Base Information
Edit
- Chemical Name:Cobalt(II) nitrate hexahydrate
- CAS No.:10026-22-9
- Molecular Formula:Co.(NO3)2.6(H2O)
- Molecular Weight:291.07
- Hs Code.:28342920
- ICSC Number:0784
- UNII:2H2166872F
- DSSTox Substance ID:DTXSID3073135
- Wikidata:Q27159001
- Mol file:10026-22-9.mol
Synonyms:cobalt nitrate;cobaltous nitrate;cobaltous nitrate dihydrate;cobaltous nitrate dodecahydrate;cobaltous nitrate hexahydrate;cobaltous nitrate nonahydrate;cobaltous nitrate tetrahydrate;cobaltous nitrate trihydrate

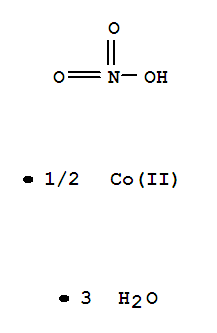

 O,
O, Xn,
Xn, N
N
