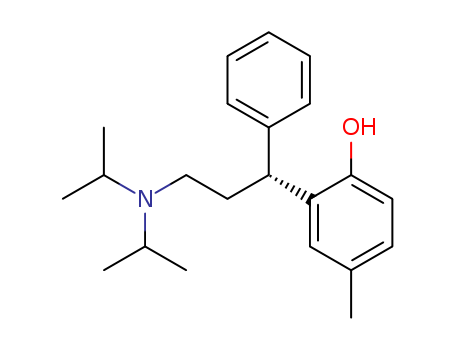
- Chemical Name:Tolterodine
- CAS No.:124937-51-5
- Deprecated CAS:215929-30-9
- Molecular Formula:C22H31NO
- Molecular Weight:325.494
- Hs Code.:
- European Community (EC) Number:805-304-4
- UNII:WHE7A56U7K
- DSSTox Substance ID:DTXSID3023687
- Nikkaji Number:J561.859B
- Wikipedia:Tolterodine
- Wikidata:Q424312
- NCI Thesaurus Code:C62083
- RXCUI:119565
- Pharos Ligand ID:39LQRXW2W8BV
- Metabolomics Workbench ID:43278
- ChEMBL ID:CHEMBL1382
- Mol file:124937-51-5.mol
Synonyms:Detrol;Detrol LA;Detrusitol;PHA 686464B;PHA-686464B;PHA686464B;Tartrate, Tolterodine;tolterodine;tolterodine tartrate;Unidet;Urotrol