10.1021/jo00907a012
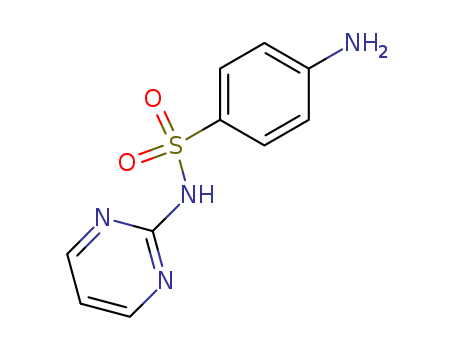
The research investigates the scope and limitations of the dimethyl sulfoxide-trifluoroacetic anhydride (DMSO-TFAA) reagent for the preparation of iminosulfuranes. The study aims to explore the efficiency and applicability of this reagent with various nitrogen-containing compounds, including aryl amines, amides, sulfonamides, and urea. The key chemicals used in the research are DMSO and TFAA, which form an intermediate reagent that reacts with the nitrogen compounds to produce iminosulfuranes. The research concludes that the DMSO-TFAA reagent is highly effective, yielding iminosulfuranes in 40-90% yields, and is particularly reactive even with aromatic amines containing certain ortho substituents. The study also highlights the reagent's ability to form iminosulfuranes from previously uncharacterized compounds like sulfanilamide and sulfadiazine. However, it notes limitations with relatively basic amines and certain aromatic compounds. The findings suggest that the DMSO-TFAA reagent is a valuable tool for the preparation of iminosulfuranes, offering a more efficient and versatile alternative to other activated DMSO reagents.


 Xn,
Xn, Xi
Xi


