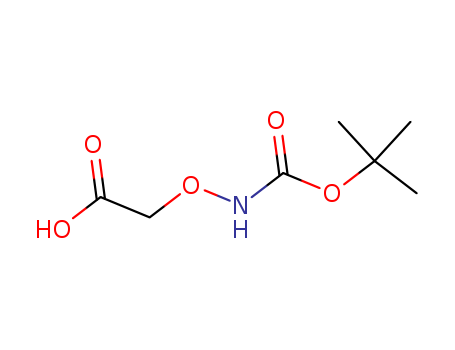
- Chemical Name:(Boc-aminooxy)acetic acid
- CAS No.:42989-85-5
- Molecular Formula:C7H13NO5
- Molecular Weight:191.184
- Hs Code.:2928 00 90
- European Community (EC) Number:628-980-0
- DSSTox Substance ID:DTXSID10373171
- Nikkaji Number:J1.289.261F
- Wikidata:Q72448316
- Mol file:42989-85-5.mol
Synonyms:(Boc-aminooxy)acetic acid;42989-85-5;Boc-Aoa-OH;2-(((tert-Butoxycarbonyl)amino)oxy)acetic acid;Boc-AOAc-OH;2-[(2-methylpropan-2-yl)oxycarbonylamino]oxyacetic Acid;N-Boc-(carboxymethoxy)amine;2-({[(tert-butoxy)carbonyl]amino}oxy)acetic acid;Boc-3-(aminooxy)acetic acid;MFCD01632027;Acetic acid, [[[(1,1-dimethylethoxy)carbonyl]amino]oxy]-;2-(tert-butoxycarbonylaminooxy)acetic acid;Boc-aminooxyacetic acid;2-(Boc-aminooxy)-acetic acid;N-Boc-(carboxymethoxy)-amine;Boc-(nhoac)-oh;(Boc)aminooxyacetic acid;[(tert-Butoxycarbonyl)aminooxy]acetic Acid;(Boc-aminoxy)acetic acid;2-(t-Butyloxycarbonyl-aminooxy)-acetic acid;t-Boc-aminooxyacetic Acid;SCHEMBL307685;DTXSID10373171;QBXODCKYUZNZCY-UHFFFAOYSA-N;tert-Butoxycarbonylaminooxyacetic acid;Boc-AOA Tert-Boc-aminooxyacetic acid;AKOS009156496;GS-6570;tert-butyloxycarbonylaminoxy acetic acid;N-tert-butoxycarbonyl aminooxyacetic acid;SY019091;(Boc-aminooxy)acetic acid, >=98.0% (T);2-(tert-Butyloxycarbonylaminooxy)acetic acid;2-[(tert-Butoxycarbonylamino)oxy]acetic acid;B3338;CS-0030574;FT-0662558;EN300-227480;H10114;[(1,1-Dimethylethoxy)carbonyl]aminooxyacetic acid;A850863;[(1,1-dimethylethoxy)carbonyl]aminooxy acetic acid;J-501795;J-650267


 Xi
Xi


