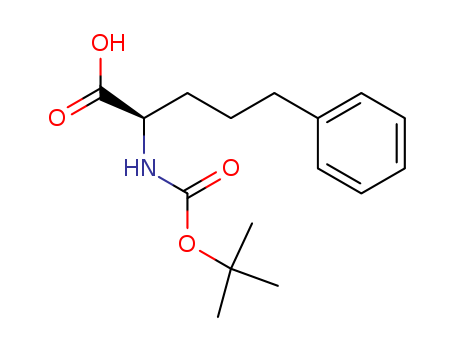
- Chemical Name:(R)-2-((tert-Butoxycarbonyl)amino)-5-phenylpentanoic acid
- CAS No.:156130-68-6
- Molecular Formula:C16H23 N O4
- Molecular Weight:293.363
- Hs Code.:2924299090
- DSSTox Substance ID:DTXSID70426874
- Nikkaji Number:J3.115.318B
- Wikidata:Q72498833
- Mol file:156130-68-6.mol
Synonyms:156130-68-6;(R)-2-((tert-Butoxycarbonyl)amino)-5-phenylpentanoic acid;(2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-5-phenylpentanoic acid;SCHEMBL2781406;DTXSID70426874;AKOS015911499;Boc-(R)-2-Amino-5-phenyl-pentanoic acid;CS-0433919;F79130;N-(tert-Butyloxycarbonyl)-5-phenyl-D-norvaline;(2R)-2-{[(tert-butoxy)carbonyl]amino}-5-phenylpentanoic acid;(2R)-2-[(1,1-dimethylethoxy)carbonyl]amino-5-phenylpentanoic acid;Benzenepentanoic acid, alpha-[[(1,1-dimethylethoxy)carbonyl]amino]-, (alphaR)-