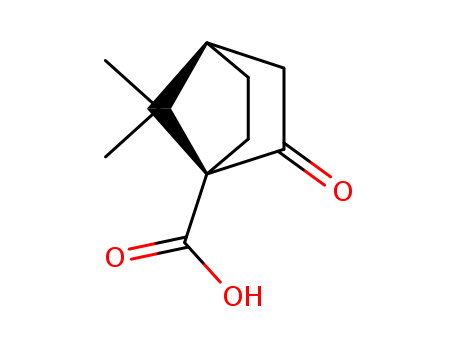
- Chemical Name:(1s,4r)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptane-1-carboxylic acid
- CAS No.:40724-67-2
- Molecular Formula:C10H14O3
- Molecular Weight:182.219
- Hs Code.:2918300090
- European Community (EC) Number:625-243-5
- Nikkaji Number:J89.375G
- Mol file:40724-67-2.mol
Synonyms:(+)-Ketopinic acid;(1S)-(+)-Ketopinic acid;40724-67-2;(1s,4r)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptane-1-carboxylic acid;(S)-(+)-Ketopinic Acid;Bicyclo[2.2.1]heptane-1-carboxylic acid, 7,7-dimethyl-2-oxo-, (1S,4R)-;S-(+)-ketopinic acid;(1S)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptane-1-carboxylic acid;AK-88562;(S)-(+)-KetopinicAcid;SCHEMBL21458724;(1S,4R)-7,7-Dimethyl-2-oxo-norbornane-1-carboxylic acid;MFCD08061242;(1S)-(+)-Ketopinic acid, 99%;AKOS006282096;CCG-208411;CS-W017111;BP-12916;DS-18484;EN300-365565;A825258;SR-05000002262;SR-05000002262-2


 Xi
Xi

