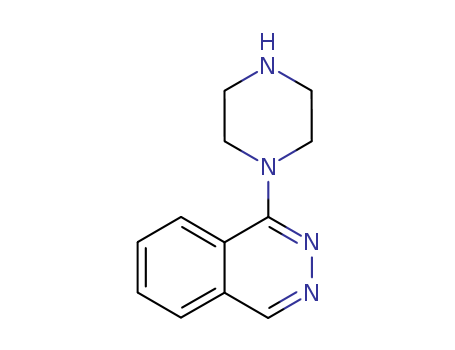
- Chemical Name:1-(Piperazin-1-yl)phthalazine
- CAS No.:118306-90-4
- Molecular Formula:C12H14 N4
- Molecular Weight:214.27
- Hs Code.:2933990090
- DSSTox Substance ID:DTXSID20561901
- Wikidata:Q82445826
- Mol file:118306-90-4.mol
Synonyms:1-(piperazin-1-yl)phthalazine;118306-90-4;1-piperazin-1-ylphthalazine;SCHEMBL2274982;DTXSID20561901;AKOS000123285;CS-0267685;EN300-43524;J-003734;F1967-2683;Z234895087