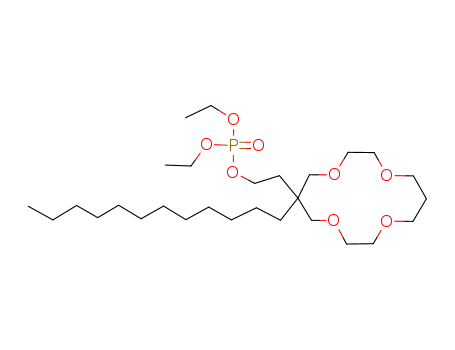
- Chemical Name:Lithium ionophore VII
- CAS No.:106868-29-5
- Molecular Formula:C28H57O8P
- Molecular Weight:552.73
- Hs Code.:2932999099
- DSSTox Substance ID:DTXSID50399931
- Nikkaji Number:J685.022G
- Wikidata:Q82202712
- Mol file:106868-29-5.mol
Synonyms:Lithium ionophore VII;106868-29-5;6-DODECYL(14-CROWN-4)-6-ETHANOL DIETHYLPHOSPHATE;2-(6-dodecyl-1,4,8,11-tetraoxacyclotetradec-6-yl)ethyl diethyl phosphate;SCHEMBL5854426;DTXSID50399931;FT-0721807;J-001667;Lithium ionophore VII, Selectophore(TM), function tested;Diethoxyphosphinic acid 2-(6-dodecyl-1,4,8,11-tetraoxacyclotetradecan-6-yl)ethyl ester