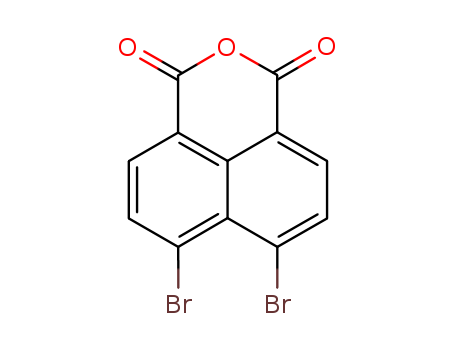
- Chemical Name:4,5-Dibromonaphthalene-1,8-dicarboxylic acid
- CAS No.:13577-26-9
- Molecular Formula:C12H4 Br2 O3
- Molecular Weight:355.97
- Hs Code.:2932999099
- DSSTox Substance ID:DTXSID10699040
- Wikidata:Q82629715
- Mol file:13577-26-9.mol
Synonyms:4,5-dibromonaphthalene-1,8-dicarboxylic acid;1H,3H-Naphtho[1,8-cd]pyran-1,3-dione,6,7-dibromo-;SCHEMBL11437330;DTXSID10699040;AC1867;CS-0334336