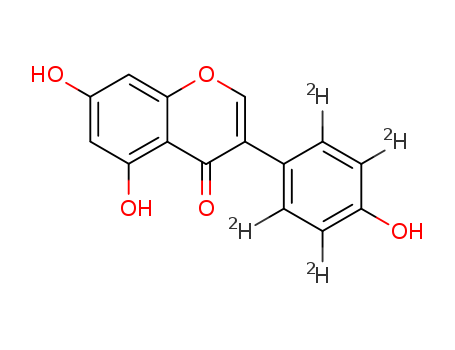
- Chemical Name:Genistein-2',3',5',6'-d4
- CAS No.:187960-08-3
- Molecular Formula:C15H6 D4 O5
- Molecular Weight:274.263
- Hs Code.:
- DSSTox Substance ID:DTXSID40473830
- Nikkaji Number:J899.253C
- Wikidata:Q82303473
- Mol file:187960-08-3.mol
Synonyms:187960-08-3;Genistein-2',3',5',6'-d4;Genistein-d4;Genistein-2',3',5',6'-d4 (Major);5,7-dihydroxy-3-(2,3,5,6-tetradeuterio-4-hydroxyphenyl)chromen-4-one;DTXSID40473830;HY-14596S;AKOS030241667;MS-23891;CS-0091916;F90926;J-012104;5,7-dihydroxy-3-[4-hydroxy(2,3,5,6-?H?)phenyl]-4H-chromen-4-one;Genistein-2 inverted exclamation mark ,3 inverted exclamation mark ,5 inverted exclamation mark ,6 inverted exclamation mark -d4 (Major)