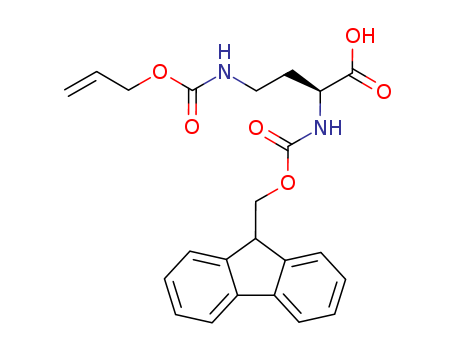
- Chemical Name:FMOC-D-DAB(ALOC)-OH
- CAS No.:387824-78-4
- Molecular Formula:C23H24N2O6
- Molecular Weight:424.453
- Hs Code.:2924299090
- Mol file:387824-78-4.mol
Synonyms:Butanoicacid, 2-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-4-[[(2-propenyloxy)carbonyl]amino]-,(2R)- (9CI)