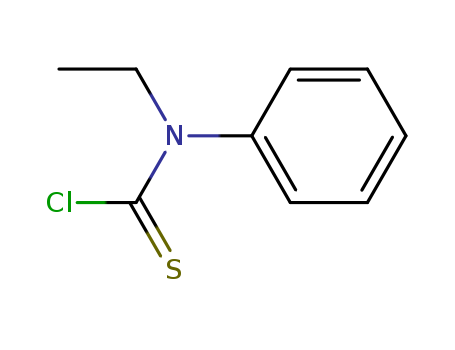
- Chemical Name:N-Ethyl-N-phenylthiocarbamoyl chloride
- CAS No.:35517-93-2
- Molecular Formula:C9H10 Cl N S
- Molecular Weight:199.704
- Hs Code.:2930909090
- European Community (EC) Number:670-602-1
- DSSTox Substance ID:DTXSID70374543
- Wikidata:Q82163160
- Mol file:35517-93-2.mol
Synonyms:N-Ethyl-N-phenylthiocarbamoyl chloride;35517-93-2;N-ethyl-N-phenylcarbamothioyl chloride;N-(CHLOROMETHANETHIOYL)-N-ETHYLANILINE;SCHEMBL11757993;DTXSID70374543;Ethyl(phenyl)carbamothioyl chloride;MFCD03093782;AKOS006276942;N-ethyl-N-phenyl-carbamothioyl chloride;A822847