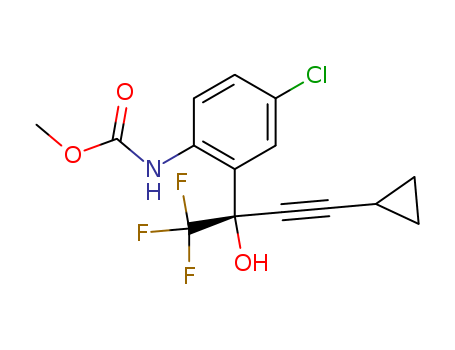
- Chemical Name:Efavirenz amino alcohol methyl carbamate
- CAS No.:211563-40-5
- Molecular Formula:C15H13 Cl F3 N O3
- Molecular Weight:347.721
- Hs Code.:
- UNII:376R1A850V
- Nikkaji Number:J1.067.510C
- Wikidata:Q27256642
- Mol file:211563-40-5.mol
Synonyms:211563-40-5;Efavirenz amino alcohol methyl carbamate;SD 573 methyl carbamate;SV-993;376R1A850V;[4-Chloro-2-[(1S)-3-cyclopropyl-1-hydroxy-1-(trifluoromethyl)-2-propynyl)phenyl]carbamic Acid Methyl Ester;UNII-376R1A850V;methyl N-[4-chloro-2-[(2S)-4-cyclopropyl-1,1,1-trifluoro-2-hydroxybut-3-yn-2-yl]phenyl]carbamate;[4-Chloro-2-[(1S)-3-cyclopropyl-1-hydroxy-1-(trifluoromethyl)-2-propynyl)phenyl]carbamic Acid Methyl;Methyl [4-Chloro-2-((1S)-1-trifluoromethyl-1-hydroxy-3-cyclopropyl-2-propyn-1-yl)phenyl]carbamate;METHYL (4-CHLORO-2-((1S)-1-TRIFLUOROMETHYL-1-HYDROXY-3-CYCLOPROPYL-2-PROPYN-1-YL)PHENYL)CARBAMATE;EFAVIRENZ IMPURITY I [WHO-IP];A899518;J-013888;Q27256642;EFAVIRENZ AMINO ALCOHOL METHYL CARBAMATE [USP IMPURITY];EFAVIRENZ IMPURITY, EFAVIRENZ AMINO ALCOHOL METHYL CARBAMATE-;(S)-METHYL 4-CHLORO-2-(4-CYCLOPROPYL-1,1,1-TRIFLUORO-2-HYDROXYBUT-3-YN-2-YL)PHENYLCARBAMATE;CARBAMIC ACID, (4-CHLORO-2-((1S)-3-CYCLOPROPYL-1-HYDROXY-1-(TRIFLUOROMETHYL)-2-PROPYNYL)PHENYL)-, METHYL ESTER;CARBAMIC ACID, N-(4-CHLORO-2-((1S)-3-CYCLOPROPYL-1-HYDROXY-1-(TRIFLUOROMETHYL)-2-PROPYN-1-YL)PHENYL)-, METHYL ESTER