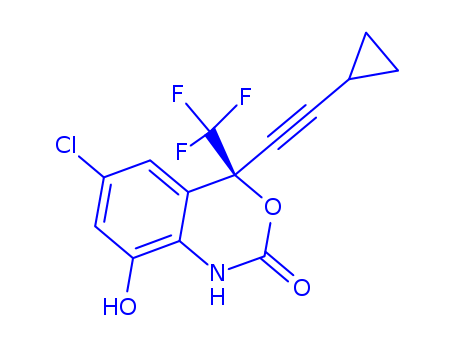
- Chemical Name:8-Hydroxyefavirenz
- CAS No.:205754-33-2
- Molecular Formula:C14H9ClF3NO3
- Molecular Weight:331.679
- Hs Code.:
- UNII:P8S49CKH6L
- DSSTox Substance ID:DTXSID50942769
- Nikkaji Number:J1.525.087I
- ChEMBL ID:CHEMBL1309
- Mol file:205754-33-2.mol
Synonyms:6-chloro-4-(cyclopropylethynyl)-8-hydroxy-4-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-2-one;8-hydroxy-efavirenz;8-hydroxy-efavirenz-glucuronide;8-hydroxyefavirenz