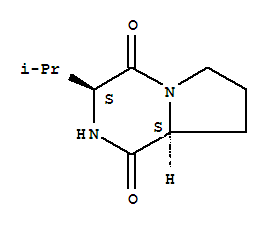
Suppliers and Price of cyclo(L-Pro-L-Val)
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- TRC
- Cyclo(L-prolyl-L-valyl)
- 25mg
- $ 335.00
- DC Chemicals
- Cyclo(L-Pro-L-Val) >98%
- 1 g
- $ 800.00
- DC Chemicals
- Cyclo(L-Pro-L-Val) >98%
- 250 mg
- $ 400.00
- Crysdot
- (3S,8AS)-3-isopropylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione 97%
- 1g
- $ 470.00
- Cayman Chemical
- Cyclo(L-Pro-L-Val)
- 50mg
- $ 55.00
- Cayman Chemical
- Cyclo(L-Pro-L-Val)
- 25mg
- $ 29.00
- Cayman Chemical
- Cyclo(L-Pro-L-Val)
- 100mg
- $ 104.00
- Cayman Chemical
- Cyclo(L-Pro-L-Val)
- 250mg
- $ 232.00
- Biosynth Carbosynth
- Cyclo(-Pro-Val)
- 100 mg
- $ 300.00
- Biosynth Carbosynth
- Cyclo(-Pro-Val)
- 50 mg
- $ 200.00
-
Total 18 raw suppliers