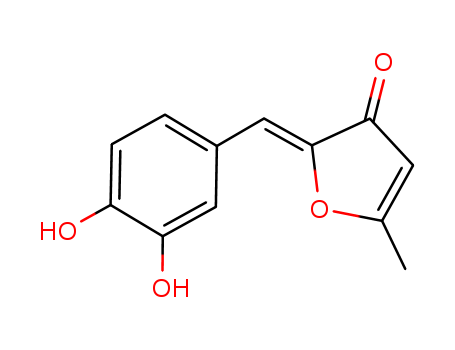
Suppliers and Price of Inotilone
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- Usbiological
- Inotilone
- 5mg
- $ 446.00
- TRC
- Inotilone
- 5mg
- $ 155.00
- Medical Isotopes, Inc.
- Inotilone
- 25 mg
- $ 2120.00
- Cayman Chemical
- Inotilone ≥98%
- 25mg
- $ 568.00
- Cayman Chemical
- Inotilone ≥98%
- 5mg
- $ 142.00
- Cayman Chemical
- Inotilone ≥98%
- 1mg
- $ 39.00
- Cayman Chemical
- Inotilone ≥98%
- 10mg
- $ 266.00
- ApexBio Technology
- Inotilone
- 5mg
- $ 182.00
- ApexBio Technology
- Inotilone
- 25mg
- $ 728.00
- ApexBio Technology
- Inotilone
- 10mg
- $ 339.00
-
Total 13 raw suppliers