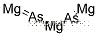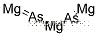Chemical Property of CID 15688015
Edit
Chemical Property:
- Melting Point:800°C
- Boiling Point:°Cat760mmHg
- Flash Point:°C
- PSA:0.00000
- Density:g/cm3
- LogP:-0.53660
- Sensitive.:Moisture Sensitive
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:0
- Rotatable Bond Count:0
- Exact Mass:98.906636
- Heavy Atom Count:2
- Complexity:2
- Purity/Quality:
-
99.9% *data from raw suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Useful:
- Canonical SMILES:[Mg]=[As]
-
Description
Magnesium arsenide has the molecular formula of
Mg3As2 and the molecular weight of 223.6592 g/mol.
It may be prepared by direct reaction between the
elements. Since As sublimes at 614°C and Mg melts at
650°C, the latter is melted at about 675°C in an inert
atmosphere like argon (not N2 which would form
nitrides) and arsenic vapors are carried into the reaction
zone by a stream of Ar gas:
3Mg + 2As + heat ? Mg3As2
Mg3As2 is obtained by this method as a deep-brown,
X-ray amorphous powder. Magnesium arsenide has the
CAS number of 12044-49-4. This compound decomposes
in contact with water:
Mg3As2 + 3H2O ? 2AsH3 + 3Mg(OH)2
Thus, it must be stored under dry conditions. Its
density is 3.15 g/cm3. This compound is dimorphic
with the low temperature form, β-Mg3As2 being cubic
(α = 12.33?) and the high-temperature form, α-Mg3As2
being hexagonal. It crystallizes in the anti-La2O3 crystal
structure, with unit-cell parameters of: a = 4.264A ° ,
c = 6.738?, c/a = 1.580. Both of these compounds are
semiconductors. The latter has a band gap, Eg, of 2.2eV
(564 nm).
-
Uses
The structure of magnesium
arsenide is not ionic but covalent.
Magnesium arsenide was used as a rodenticide and
insecticide. Its toxicity to humans has led to its prohibition
in the US and Europe.