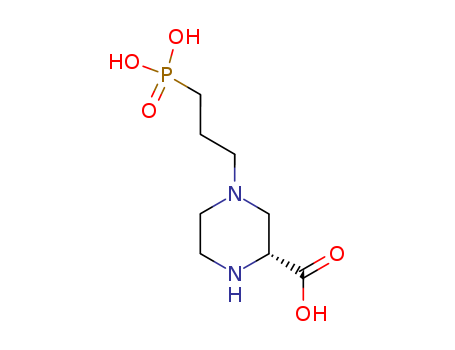
- Chemical Name:(2R)-4-(3-phosphonopropyl)piperazine-2-carboxylic acid
- CAS No.:126453-07-4
- Molecular Formula:C8H17N2O5P
- Molecular Weight:252.207
- Hs Code.:
- UNII:A3QV2VT7SN
- DSSTox Substance ID:DTXSID501208909
- Nikkaji Number:J259.993G
- ChEMBL ID:CHEMBL47277
- Mol file:126453-07-4.mol
Synonyms:(R)-CPP;126453-07-4;(2R)-4-(3-phosphonopropyl)piperazine-2-carboxylic acid;(R)-4-(3-phosphonopropyl)piperazine-2-carboxylic acid;A3QV2VT7SN;CHEMBL47277;2-Piperazinecarboxylic acid, 4-(3-phosphonopropyl)-, (2R)-;CPP, (R)-;CPP, (-)-;3-((R)-2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid;3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid, (R)-;7RC;Tocris-0173;Tocris-0247;Lopac-C-104;UNII-A3QV2VT7SN;SCHEMBL1557957;(-)-CPP;CUVGUPIVTLGRGI-SSDOTTSWSA-N;DTXSID501208909;BDBM50050704;HB0021;AKOS024457118;NCGC00015179-01;NCGC00015179-02;NCGC00024482-01;NCGC00024515-01;BC168104;HY-100814;LS-110988;CS-0020456;SR-01000597718;J-005384;SR-01000597718-1;(R)-4-(3-phosphonopropyl)piperazine-2-carboxylicacid;(2R)-4-(3-Phosphonopropyl)-2-piperazinecarboxylic acid;(R)-4-(3-Phosphono-propyl)-piperazine-2-carboxylic acid;3-((R)-Carboxylpiperazin-4-yl)-propyl-1-phosphonic acid