Base Information
Edit
- Chemical Name:Apixaban
- CAS No.:503612-47-3
- Molecular Formula:C25H25N5O4
- Molecular Weight:459.50
- Hs Code.:
- European Community (EC) Number:639-684-6
- UNII:3Z9Y7UWC1J
- DSSTox Substance ID:DTXSID80436500
- Nikkaji Number:J2.566.952E
- Wikipedia:Apixaban
- Wikidata:Q414462
- NCI Thesaurus Code:C61308
- RXCUI:1364430
- Pharos Ligand ID:K9R389ZL42D1
- Metabolomics Workbench ID:65917
- ChEMBL ID:CHEMBL231779
- Mol file:503612-47-3.mol
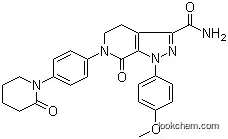
Synonyms:apixaban;BMS 562247;BMS-562247;BMS-562247-01;BMS562247;Eliquis


