10.1039/c39930000689
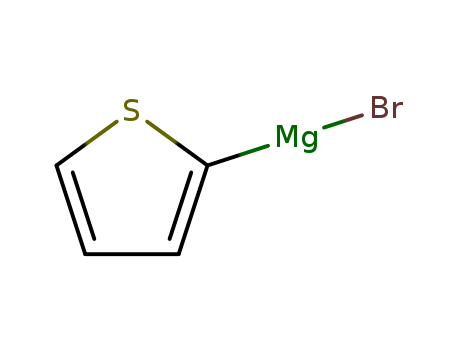
This research focuses on the development of new n-electron donors by combining the tetrathiafulvalene (TTF) system with conjugated backbones of substituted terthienyls to improve control of charge transport and superconducting properties of the corresponding cation radical salts (CRSs). The study aims to increase material dimensionality by modifying the spatial extension of the n-donor and intermolecular interstack bonding. The researchers synthesized extended π-electron donors of the TTF series containing substituted terthienyl spacers using a five-step synthetic route. Key chemicals used include 3-octyl and 3-(3,6-dioxaheptyl)thiophene, bromine, 2-thienylmagnesium bromide, N-methylformanilide, acetic anhydride, and phosphonic acid. The study found that substitution of the conjugated spacer significantly affects the solubility, electronic absorption spectrum, and electrochemical behavior of the donor molecules. For example, the introduction of methyl groups increases solubility, while longer alkyl or oxyalkyl substituents cause red shifts in the absorption spectrum. The terthienyl spacer also leads to a marked decrease in oxidation potentials and a reduction in on-site coulombic repulsion between positive charges in the dication state. The research concludes that these new structures can be considered as first examples of oligothiophenes derivatized by a TTF system, and further work is needed to clarify the mechanisms behind the observed spectral changes and to explore the electrical properties of the conducting adducts obtained by iodine doping.