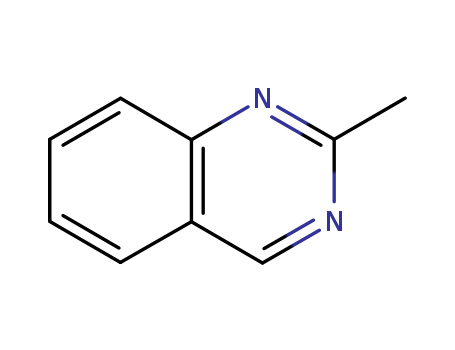
- Chemical Name:2-Methylquinazoline
- CAS No.:700-79-8
- Molecular Formula:C8H5 Cl N2
- Molecular Weight:144.176
- Hs Code.:
- DSSTox Substance ID:DTXSID001315600
- Nikkaji Number:J101.312B
- ChEMBL ID:CHEMBL3287981
- Mol file:700-79-8.mol
Synonyms:2-Methylquinazoline;700-79-8;Quinazoline, 2-methyl-;Quinazoline, 2-methyl- (6CI,7CI,8CI,9CI);2-Methyl-quinazoline;methylquinazoline;SCHEMBL196151;CHEMBL3287981;DTXSID001315600;AS-0206;CS-0099614