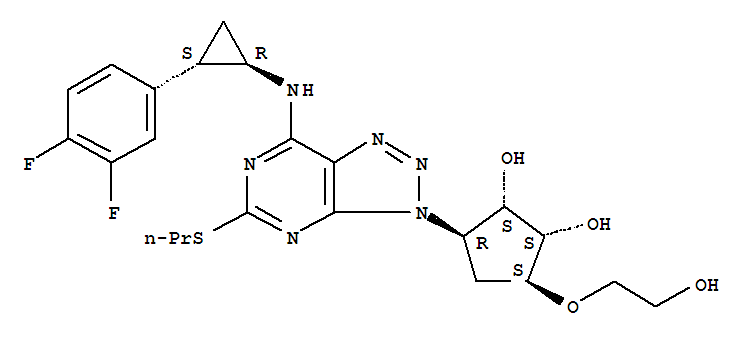
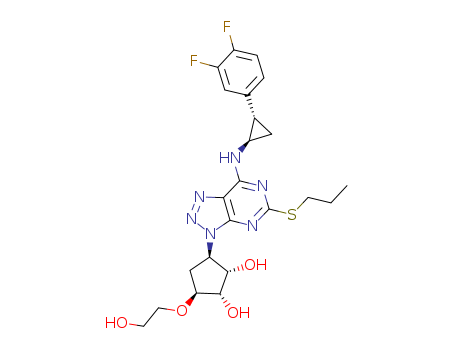
- Chemical Name:Ticagrelor
- CAS No.:274693-27-5
- Molecular Formula:C23H28F2N6O4S
- Molecular Weight:522.576
- Hs Code.:29335990
- European Community (EC) Number:619-540-9
- UNII:GLH0314RVC
- DSSTox Substance ID:DTXSID901009337
- Nikkaji Number:J1.905.358J
- Wikipedia:Ticagrelor
- Wikidata:Q420542
- NCI Thesaurus Code:C76404
- RXCUI:1116632
- Pharos Ligand ID:2AXUW7HYJT92
- Metabolomics Workbench ID:43727
- ChEMBL ID:CHEMBL398435
- Mol file:274693-27-5.mol
Synonyms:3-(7-((2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-(1-3)-triazolo(4,5-d)pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol;AZD 6140;AZD-6140;AZD6140;Brilinta;Brilique;Ticagrelor