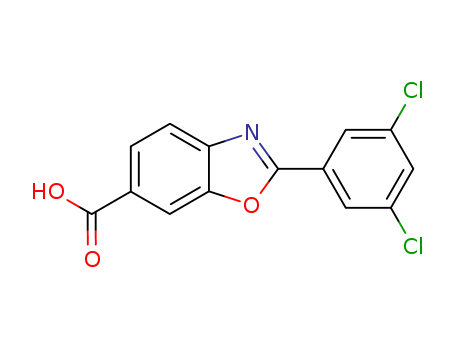
- Chemical Name:Tafamidis
- CAS No.:594839-88-0
- Molecular Formula:C14H7Cl2NO3
- Molecular Weight:308.12
- Hs Code.:
- European Community (EC) Number:813-715-5
- UNII:8FG9H9D31J
- DSSTox Substance ID:DTXSID00208185
- Nikkaji Number:J1.930.213J
- Wikipedia:Tafamidis
- Wikidata:Q519447
- NCI Thesaurus Code:C84193
- RXCUI:1545063
- Pharos Ligand ID:45CWA8GX7BAP
- Metabolomics Workbench ID:152765
- ChEMBL ID:CHEMBL2103837
- Mol file:594839-88-0.mol
Synonyms:FX 1006A;FX-1006A;FX1006A;tafamidis;tafamidis meglumine;Vyndamax;Vyndaqel