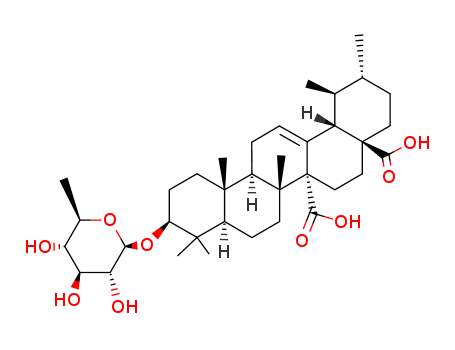
- Chemical Name:Quinovin
- CAS No.:107870-05-3
- Molecular Formula:C36H56O9
- Molecular Weight:632.835
- Hs Code.:
- UNII:5655DJH94B
- Nikkaji Number:J397.834F
- Wikidata:Q27261375
- Mol file:107870-05-3.mol
Synonyms:Quinovin;107870-05-3;Chinovin;Quinovin [MI];Quinova-bitter;Quivin;5655DJH94B;Quinovic acid 3-O-|A-D-quinovopyranoside;(1S,2R,4aS,6aR,6aR,6bR,8aR,10S,12aR,14bS)-1,2,6b,9,9,12a-hexamethyl-10-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a,6a-dicarboxylic acid;53516-73-7;UNII-5655DJH94B;3-O-(beta-D-Quinovopyranosyl)quinovic acid;HY-N1528;AKOS032948561;URS-12-ENE-27,28-dioic acid, 3-((6-deoxy-beta-D-glucopyranosyl)oxy)-, (3beta)-;FS-10116;CS-0017077;Cinchonaglycoside A, >=95% (LC/MS-ELSD);3-O-(.BETA.-D-QUINOVOPYRANOSYL)QUINOVIC ACID;Q27261375;URS-12-ENE-27,28-DIOIC ACID, 3-((6-DEOXY-.BETA.-D-GLUCOPYRANOSYL)OXY)-, (3.BETA.)-