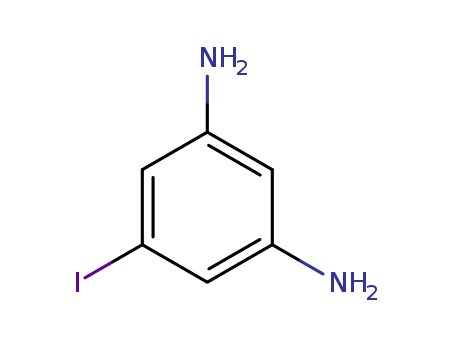
- Chemical Name:5-Iodobenzene-1,3-diamine
- CAS No.:111938-17-1
- Molecular Formula:C6H7IN2
- Molecular Weight:234.039
- Hs Code.:
- NSC Number:80185
- DSSTox Substance ID:DTXSID00292064
- Nikkaji Number:J1.437.212A
- Wikidata:Q82030206
- Mol file:111938-17-1.mol
Synonyms:5-iodobenzene-1,3-diamine;111938-17-1;5-Iodo-benzene-1,3-diamine;1,3-Benzenediamine, 5-iodo-;5-iodo-1,3-phenylenediamine;NSC80185;NCIOpen2_004408;SCHEMBL8952443;DTXSID00292064;JYDAWJKJURFTTH-UHFFFAOYSA-N;NSC-80185;AKOS004121689;SB81254;CS-0239138;FT-0709175;EN300-206543;F87692;Z1269210269