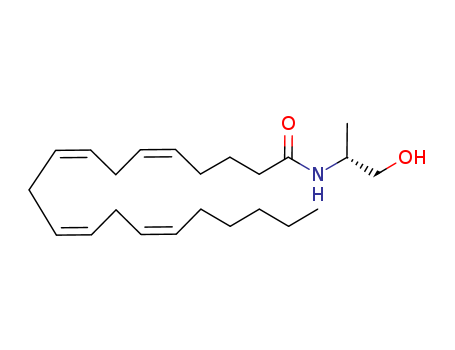
- Chemical Name:N-[(2R)-1-hydroxypropan-2-yl]eicosa-5,8,11,14-tetraenamide
- CAS No.:157182-49-5
- Molecular Formula:C23H39 N O2
- Molecular Weight:361.5613
- Hs Code.:
- DSSTox Substance ID:DTXSID70425004
- Wikipedia:Methanandamide
- Wikidata:Q27194429
- Mol file:157182-49-5.mol
Synonyms:157182-49-5;N-[(2R)-1-hydroxypropan-2-yl]eicosa-5,8,11,14-tetraenamide;R(+)-Methanandamide;CBiol_001724;CBiol_002048;KBioGR_000005;KBioSS_000005;KBio2_000005;KBio2_002573;KBio2_005141;KBio3_000009;KBio3_000010;DTXSID70425004;Bio1_000010;Bio1_000334;Bio1_000499;Bio1_000823;Bio1_000988;Bio1_001312;Bio2_000005;Bio2_000485;N-[(2R)-1-hydroxypropan-2-yl]icosa-5,8,11,14-tetraenamide;PD133429;Q27194429


 F
F


