- Sulfuryl chloride
- Sulfuryl chloride fluoride
- Sulfuryl fluoride
- 7791-28-8Bariumbromide (BaBr2), dihydrate (9CI)
- 7791-49-32,1,3-Benzoxadiazole,5-methoxy-, 1-oxide
- 77916-77-9Hexanedioicacid, 1,6-bis(1-butylpentyl) ester
- 7791-71-1Thymidine,5'-O-(triphenylmethyl)-
- 77-91-8Choline dihydrogencitrate salt
- 779-26-0Ethanone,1-(2-biphenylenyl)-
- 779-27-12H-1-Benzopyran-3-carboxylicacid, 7-hydroxy-2-oxo-
- 779286-44-12-(Trifluoromethyl)phenylacetamide
- 77-92-9Citric acid
- 77-93-01,2,3-Propanetricarboxylicacid, 2-hydroxy-, 1,2,3-triethyl ester
Hot Products
- 30931-67-06-Benzothiazolesulfonicacid, 2,2'-(1,2-hydrazinediylidene)bis[3-ethyl-2,3-dihydro-, ammonium salt(1:2)
- 12124-97-9Ammonium bromide
- 142-47-2L-Glutamic acid, sodiumsalt (1:1)
- 66357-35-5Ranitidine hydrochloride
- 101-83-7Cyclohexanamine,N-cyclohexyl-
- 482-44-0Imperatorin
- 4635-59-0Butanoylchloride, 4-chloro-
- 75-64-92-Propanamine,2-methyl-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Sulfuryl chloride |
EINECS | 232-245-6 |
| CAS No. | 7791-25-5 | Density | 1.865 g/cm3 |
| PSA | 42.52000 | LogP | 1.78960 |
| Solubility | reacts with water | Melting Point |
-54 °C |
| Formula | Cl2O2S | Boiling Point | 69.1 °C at 760 mmHg |
| Molecular Weight | 134.971 | Flash Point | 69.1 °C |
| Transport Information | UN 2927 6.1/PG 2 | Appearance | colourless or pale yellow li |
| Safety | 26-45-36/37-36/37/39 | Risk Codes | 34-40-14-37 |
| Molecular Structure |
|
Hazard Symbols |
 C C
|
| Synonyms |
Sulfonylchloride;Sulfonyl dichloride;Sulfur chloride oxide (SCl2O2);Sulfur chlorideoxide (SO2Cl2);Sulfur oxychloride (SO2Cl2);Sulfuric dichloride;Sulfuricoxychloride;Sulfuryl chloride (SO2Cl2);Sulfuryl dichloride;Sulphurylchloride; |
Article Data | 107 |
Sulfuryl chloride Chemical Properties
The molecular structure of Sulfuryl chloride (CAS NO.7791-25-5):
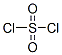
IUPAC Name: Sulfuryl dichloride
Molecular Weight: 134.9698 g/mol
Molecular Formula: Cl2O2S
Density: 1.865 g/cm3
Melting Point: -54.1 °C
Boiling Point: 69.1 °C at 760 mmHg
Flash Point: 69.1 °C
Index of Refraction: 1.479
Molar Refractivity: 20.52 cm3
Molar Volume: 72.3 cm3
Surface Tension: 49.8 dyne/cm
Enthalpy of Vaporization: 31.4 kJ/mol
Vapour Pressure: 148 mmHg at 25 °C
Water Solubility: reacts
Sensitive: moisture sensitive
XLogP3-AA: 1.2
H-Bond Acceptor: 2
Exact Mass: 133.899605
MonoIsotopic Mass: 133.899605
Topological Polar Surface Area: 34.1
Heavy Atom Count: 5
Canonical SMILES: O=S(=O)(Cl)Cl
InChI: InChI=1S/Cl2O2S/c1-5(2,3)4
EINECS: 232-245-6
Product Categories: Inorganics; Chlorination; C-X Bond Formation (Halogen); Synthetic Reagents; ChlorinationMicro/Nanoelectronics; Electronic Chemicals; Others
Sulfuryl chloride Uses
Sulfuryl chloride (CAS NO.7791-25-5) is widely used as a reagent in the conversion of C-H → C-Cl adjacent to activating substituents such as carbonyls and sulfoxides. It is often used as source of Cl2. SO2Cl2. Sulfuryl chloride can also convert alcohols to alkyl chlorides. In industry, sulfuryl chloride is most used in producing pesticides. It can also be used to treat wool to prevent shrinking.
Sulfuryl chloride Production
Sulfuryl chloride is made by the reaction of sulfur dioxide and chlorine in the presence of a catalyst, such as activated carbon.
SO2 + Cl2 → SO2Cl2
The crude product can be purified by fractional distillation. It is uncommon to prepare SO2Cl2 in the laboratory because it is commercially available.
Sulfuryl chloride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LC50 | inhalation | 159ppm/4H (159ppm) | Toxicologist. Vol. 3, Pg. 62, 1983. |
Sulfuryl chloride Consensus Reports
Reported in EPA TSCA Inventory.
Sulfuryl chloride Safety Profile
Hazard Codes:  C
C
Risk Statements: 34-40-14-37
R34:Causes burns.
R40:Limited evidence of a carcinogenic effect.
R14 :Reacts violently with water.
R37:Irritating to respiratory system
Safety Statements: 26-45-36/37-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37:Wear suitable protective clothing and gloves.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
RIDADR: UN 2927 6.1/PG 2
WGK Germany: 2
RTECS: WT4870000
F: 3-10-21
HazardClass: 8
PackingGroup: I
HS Code: 28121099
A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental tumorigenic data. Can explode with PbO2. Will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with alkalies, diethyl ether, dimethyl sulfoxide, dinitrogen pentaoxide, lead dioxide, phosphorus. When heated to decomposition it emits highly toxic fumes of Cl− and SOx. See also SULFURIC ACID and HYDROCHLORIC ACID, which are formed upon hydrolysis.
Sulfuryl chloride Standards and Recommendations
DOT Classification: 8; Label: Corrosive, Poison
Sulfuryl chloride Specification
Sulfuryl chloride (CAS NO.7791-25-5) is also named as Caswell No. 816 ; EPA Pesticide Chemical Code 078002 ; HSDB 827 ; Sulfonyl chloride ; Sulfonyl dichloride ; Sulfur oxychloride (SO2Cl2) ; Sulfuric dichloride ; Sulfuric oxychloride ; Sulfuryl chloride (SO2Cl2) ; Sulfuryl dichloride ; Sulphuryl chloride . Sulfuryl chloride (CAS NO.7791-25-5) is colourless or pale yellow liquid with a pungent odor. It is very toxic by inhalation and corrosive to metals and tissue. Sulfuryl chloride reacts exothermically with water.It is incompatible with strong oxidizing agents, alcohols, amines. It reacts violently with bases and attacks many metals. It can react explosively with lead dioxide. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts. Vapors cause severe irritation of eyes and respiratory system. Liquid burns eyes and skin. If ingested, can cause severe burns of mouth and stomach. Toxic and irritating gases are generated in fire.

