|
Name
|
|
EINECS
|
200-720-7
|
|
CAS No.
|
69-93-2
|
Density
|
1.87 g/cm3
|
|
PSA
|
114.37000
|
LogP
|
-1.76720
|
|
Solubility
|
Soluble in 1M sodium hydroxide solution. Slightly soluble in water. Insoluble in ether and alcohol.
|
Melting Point
|
>300°C(lit.)
|
|
Formula
|
C5H4N4O3
|
Boiling Point
|
863 °C at 760 mmHg
|
|
Molecular Weight
|
168.112
|
Flash Point
|
475.7 °C
|
|
Transport Information
|
N/A
|
Appearance
|
Off-white crystals
|
|
Safety
|
24/25-36-26
|
Risk Codes
|
33-36/37/38
|
|
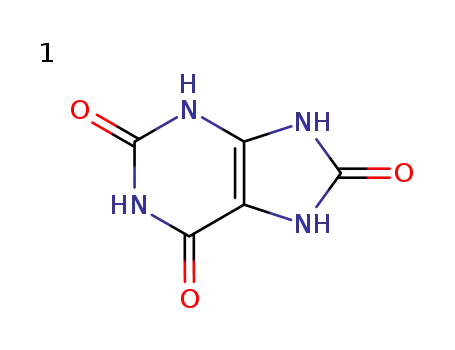
Molecular Structure
|
|
Hazard Symbols
|
 Xi Xi
|
|
Synonyms
|
Uric acid(8CI);1H-Purine-2,6,8-triol;2,6,8-Trihydroxypurine;2,6,8-Trioxopurine;2,6,8-Trioxypurine;Lithic acid;NSC 3975;Purine-2,6,8(1H,3H,9H)-trione;1H-Purine-2,6,8 (3H)-trione, 7,9-dihydro-;1H-purine-2,6,8(3H)-trione, 7,9-dihydro-;1H-Purine-2,6,8(3H)-trione, 7,9-dihydro- (9CI);2,6,8-trioxopurine;
|
Article Data |
120
|
The Uric acid, with the CAS registry number 69-93-2 and EINECS registry number 200-720-7, has the systematic name of 7,9-dihydro-1H-purine-2,6,8(3H)-trione. It is a kind of off-white crystals, and the molecular formula of this chemical is C5H4N4O3.
The Uric acid is created when the body breaks down purine nucleotides. High concentrations of uric acid in blood serum can lead to a type of arthritis known as gout. About its solubilities: It is slightly soluble in water, and it exhibit greater solubility in hot water than cold, allowing for easy recrystallization. The solubility of the acid and its salts in ethanol is very low or negligible. In ethanol water mixtures, the solubilities are somewhere between the end values for pure ethanol and pure water.
The physical properties of Uric acid are as following: (1)ACD/LogP: -1.08; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.33; (4)ACD/LogD (pH 7.4): -2.67; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 3.46; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 7; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 64.17 Å2; (13)Index of Refraction: 1.721; (14)Molar Refractivity: 35.51 cm3; (15)Molar Volume: 89.7 cm3; (16)Polarizability: 14.07×10-24cm3; (17)Surface Tension: 94.3 dyne/cm; (18)Density: 1.87 g/cm3; (19)Flash Point: 475.7 °C; (20)Enthalpy of Vaporization: 129.69 kJ/mol; (21)Boiling Point: 863 °C at 760 mmHg; (22)Vapour Pressure: 2.63E-31 mmHg at 25°C.
Produce and uses of Uric acid: It can be produced by xanthine oxidase from xanthine and hypoxanthine, which in turn are produced from purine. Uric acid is released in hypoxic conditions. What's more, it is used as reagent in the determination of uricase and tungstate. With a wonderful moisture retention, it is often used in cosmetics.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin, and has danger of cumulative effects. Therefore, you had better take the following instructions: Avoid contacting with skin and eyes; Wear suitable protective clothing, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1\C2=C(/NC(=O)N1)NC(=O)N2
(2)InChI: InChI=1/C5H4N4O3/c10-3-1-2(7-4(11)6-1)8-5(12)9-3/h(H4,6,7,8,9,10,11,12)
(3)InChIKey: LEHOTFFKMJEONL-UHFFFAOYAN





 Xi
Xi












