Products Categories
| CAS No.: | 10039-54-0 |
|---|---|
| Name: | Hydroxylamine sulfate |
| Molecular Structure: | |
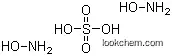 |
|
| Formula: | 2(H3NO).H2SO4 |
| Molecular Weight: | 164.14 |
| Synonyms: | Hydroxylammonium sulfate;Hydroxylamine, sulfate;Bis(hydroxylammonium) sulphate;Hydroxylamine, sulfate (2:1);Hydroxylamine sulfate [UN2865] [Corrosive];Hydroxylamine sulphate;Hydroxylamine, sulfate (2:1) (salt);Hydroxylamine neutral sulfate;hydroxyazanium sulfate;Hydroxylamine sulfate (2:1);Bis(hydroxylamine) sulfate;Hydroxylaminsulfate;Hydroxylamin Sulfat;hydroxylammonium sulphate; |
| EINECS: | 233-118-8 |
| Density: | 1.86 g/cm3 |
| Melting Point: | 170 °C (dec.)(lit.) |
| Boiling Point: | 330 °C at 760 mmHg |
| Solubility: | 329 g/L (20 °C) in water |
| Appearance: | white crystals or powder |
| Hazard Symbols: |
 Xn, Xn,  N N
|
| Risk Codes: | 22-36/38-43-48/22-50 |
| Safety: | 22-24-37-61 |
| Transport Information: | UN 2865 8/PG 3 |
| PSA: | 175.48000 |
| LogP: | 0.49720 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- 108-78-1Melamine
- 74-79-3L(+)-Arginine
- 9004-54-0Dextran
- 107-35-7Taurine
- 73-31-4Melatonine
- 157115-85-0Noopept
- 27247-96-7Nitric acid,2-ethylhexyl ester
- 9002-89-5Ethenol, homopolymer
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Chemistry
The molecular structure of Hydroxylamine sulfate (CAS NO.10039-54-0):

IUPAC Name: Hydroxyazanium sulfate
Molecular Weight: 164.13832 g/mol
Molecular Formula: H8N2O6S
Density: 1.86 g/cm3
Melting Point: 170 °C (dec.)(lit.)
Enthalpy of Vaporization: 62.94 kJ/mol
Boiling Point: 330 °C at 760 mmHg
Vapour Pressure: 3.35E-05 mmHg at 25 °C
Water Solubility: 329 g/L (20 °C)
H-Bond Donor: 4
H-Bond Acceptor: 6
Exact Mass: 164.010307
MonoIsotopic Mass: 164.010307
Topological Polar Surface Area: 176
Heavy Atom Count: 9
Canonical SMILES: [NH3+]O.[NH3+]O.[O-]S(=O)(=O)[O-]
InChI: InChI=1S/2H4NO.H2O4S/c2*1-2;1-5(2,3)4/h2*2H,1H3;(H2,1,2,3,4)/q2*+1;/p-2
InChIKey: VGYYSIDKAKXZEE-UHFFFAOYSA-L
EINECS: 233-118-8
Product Categories: Pharmaceutical Intermediates; Anilines, Aromatic Amines and Nitro Compounds; Inorganics; Hydroxylamines (Unsubstituted); Amination; Hydroxylamines; Synthetic Organic Chemistry
Uses
Hydroxylamine sulfate (CAS NO.10039-54-0) is used in organic synthesis. It is also used to generate hydroxylamine-O-sulfonic acid from oleum or chlorosulfuric acid. It is used in photography as a stabiliser for colour developers and as an additive in photographic emulsions in colour film. Hydroxylammonium sulfate is used in the production of anti-skinning agents, pharmaceuticals, rubber, textiles, plastics and detergents. It is a radical scavenger that terminates radical polymerization reactions and serves as an antioxidant in natural rubber. (NH3OH)2SO4 is a starting material for some insecticides, herbicides and growth regulators.
Production
Hydroxylammonium sulfate can be obtained by the acid-base reaction of hydroxylamine with sulfuric acid:
2NH2OH(aq) + H2SO4(aq) → (NH3OH)2SO4(aq)
Toxicity Data With Reference
| 1. | cyt-dmg-orl 5000 ppm | CARYAB Caryologia. 31 (1978),1. | ||
| 2. | ipr-mus LDLo:102 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 23 (1972),288. | ||
| 3. | skn-rbt LDLo:100 mg/kg | FAATDF Fundamental and Applied Toxicology. 8 (1987),583. |
Consensus Reports
Reported in EPA TSCA Inventory.
Safety Profile
Hazard Codes:  Xn,
Xn,  N
N
Risk Statements: 22-36/38-43-48/22-50
R22:Harmful if swallowed.
R36/38:Irritating to eyes and skin.
R43:May cause sensitization by skin contact.
R48/22:Harmful: danger of serious damage to health by prolonged exposure if swallowed.
R50:Very toxic to aquatic organisms.
Safety Statements: 22-24-37-61
S22:Do not breathe dust.
S24:Avoid contact with skin.
S37:Wear suitable gloves.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 2865 8/PG 3
WGK Germany: 3
RTECS: NC5425000
F: 21
HazardClass: 8
PackingGroup: III
HS Code: 28251000
Poison by skin contact and intraperitoneal routes. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Moderately explosive when exposed to heat or by chemical reaction. In the presence of alkalies at elevated temperatures, free hydroxylamine is liberated and may decompose explosively. When heated to decomposition it emits toxic fumes of SOx and NOx. See also AMINES and SULFATES.
Standards and Recommendations
DOT Classification: 8; Label: Corrosive
Specification
Hydroxylamine sulfate (CAS NO.10039-54-0) is also named as Bis(hydroxylamine) sulfate ; Hydroxylamine neutral sulfate ; Hydroxylamine sulfate (2:1) ; Hydroxylamine, sulfate ; Oxammonium sulfate . Hydroxylamine sulfate (CAS NO.10039-54-0) is white crystals or powder. It is water soluble. Hydroxylamine sulfate may decompose explosively in the presence of alkalies. Contact may cause severe irritation to skin, eyes, and mucous membranes. It may be toxic by ingestion. Sulfuric acid fumes may form in fires. Solid Hydroxylamine sulfate explodes when heated to 170 °C. Sodium ignites on contact with hydroxylamine. Health Hazard Inhalation of dust or ingestion may cause systemic poisoning characterized by cyanosis, methemoglobinemia, convulsions, and coma. Contact with eyes or skin causes irritation.
