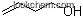Products Categories
| CAS No.: | 107-88-0 |
|---|---|
| Name: | 1,3-Butanediol |
| Article Data: | 193 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H10O2 |
| Molecular Weight: | 90.1222 |
| Synonyms: | 1,3-Butylene glycol;Butylene glycol;1,3-Dihydroxybutane;1-Methyl-1,3-propanediol;Caswell No. 128GG;beta-Butylene glycol; |
| EINECS: | 203-529-7 |
| Density: | 1.001 g/cm3 |
| Melting Point: | -54 °C |
| Boiling Point: | 207 °C at 760 mmHg |
| Flash Point: | 121.1 °C |
| Solubility: | soluble in water |
| Appearance: | colourless liquid |
| Safety: | 24/25 |
| PSA: | 40.46000 |
| LogP: | -0.25040 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- 2915-53-92-Butenedioic acid(2Z)-, 1,4-dioctyl ester
- 10097-28-6Silylene, oxo-
- 2753-45-9Benzoic acid,3,4-dimethoxy-, 4-[ethyl[2-(4-methoxyphenyl)-1-methylethyl]amino]butyl ester,hydrochloride (1:1)
- 12138-09-9Tungsten sulfide (WS2)
- 55295-98-2Guanidine,cyano-,polymer with ammonium chloride ((NH4)Cl) and formaldehyde
- 31512-74-0Poly[oxy-1,2-ethanediyl(dimethyliminio)-1,2-ethanediyl(dimethyliminio)-1,2-ethanediylchloride (1:2)]
- 772-33-8Phenol,2-(bromomethyl)-4-nitro-
- 221615-75-4Ethanone,1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
Specification
The IUPAC name of 1,3-Butanediol is butane-1,3-diol. With the CAS registry number 107-88-0 and EINECS 203-529-7, it is also named as Butylene glycol. The classification codes are Reproductive Effect; Skin / Eye Irritant. It is colourless liquid which is miscible with water, ethanol, acetone, methyl ethyl ketone and dibutyl phthalate miscible, insoluble in aliphatic hydrocarbons, benzene, toluene, carbon tetrachloride, phenol, 2-amino ethanol, mineral oil, cotton seed oil and so on. What's more, it is flammable. When heated to decomposition it emits acrid smoke and irritating fumes. So the storage environment should be well-ventilated, low-temperature and dry. Keep 1,3-Butanediol separate from oxidant.
The other characteristics of this product can be summarized as:(1)# of Rule of 5 Violations: 0; (2)ACD/LogD (pH 5.5): -0.69; (3)ACD/LogD (pH 7.4): -0.69; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 10.01; (7)ACD/KOC (pH 7.4): 10.01; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 40.46 Å2; (12)Index of Refraction: 1.437; (13)Molar Refractivity: 23.6 cm3; (14)Molar Volume: 89.9 cm3; (15)Surface Tension: 37.1 dyne/cm; (16)Enthalpy of Vaporization: 51.56 kJ/mol; (17)Vapour Pressure: 0.0541 mmHg at 25°C; (18)Rotatable Bond Count: 2; (19)Exact Mass: 90.06808; (20)MonoIsotopic Mass: 90.06808; (21)Topological Polar Surface Area: 40.5; (22)Heavy Atom Count: 6; (23)Complexity: 28.7.
Preparation of 1,3-Butanediol: It can be obtained by the condensation of propylene and formaldehyde in the presence of sulfuric acid.
Uses of 1,3-Butanediol: It is commonly used as a solvent for food flavouring agents and is a co-monomer used in certain polyurethane and polyester resins. It is also used in organic synthesis. Such as, it can react with tert-butyl-diphenylchlorosilane to get 4-(tert-butyl-diphenyl-silanyloxy)-butan-2-ol. This reaction needs reagent imidazole and solvent tetrahydrofuran at ambient temperature. The reaction time is 50 min. The yield is 93%.
.gif)
People can use the following data to convert to the molecule structure.
1. Smiles:C([C@@H](C)O)CO
2. InChI:InChI=1/C4H10O2/c1-4(6)2-3-5/h4-6H,2-3H2,1H3
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 11gm/kg (11000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 259, 1941. |
| mouse | LD50 | intraperitoneal | 10276mg/kg (10276mg/kg) | Toxicology and Applied Pharmacology. Vol. 49, Pg. 385, 1979. | |
| mouse | LD50 | oral | 12980mg/kg (12980mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 45, Pg. 669, 1956. | |
| rabbit | LD50 | skin | > 20gm/kg (20000mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 14, 1974. | |
| rat | LD50 | oral | 18610mg/kg (18610mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 259, 1941. | |
| rat | LD50 | subcutaneous | 20gm/kg (20000mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 14, 1974. |