Products Categories
| CAS No.: | 122-99-6 |
|---|---|
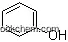
| Name: | 2-Phenoxyethanol |
| Article Data: | 122 |
| Molecular Structure: | |
|
|
|
| Formula: | C8H10O2 |
| Molecular Weight: | 138.166 |
| Synonyms: | Sepicide LD;b-Hydroxyethyl phenyl ether;b-Phenoxyethanol;b-Phenoxyethyl alcohol;(2-Hydroxyethoxy)benzene;1-Hydroxy-2-phenoxyethane;2-Hydroxyethyl phenyl ether;2-Phenoxyethyl alcohol;Arosol;Dalpad A;Dowanol EP;Dowanol EPh;Emeressence1160;Ethylene glycol monophenyl ether;Ethylene glycol phenyl ether;Euxyl PE90120;H 4644;Hisolve EPH;NSC 1864;Newpol EFP;PHE (alcohol);PHE-G;PHE-S;Phenova;Phenoxethol;Phenoxetol;Phenoxyethanol;Phenoxyethyl alcohol;Phenyl cellosolve;Plastilit DS 3431;Rokafenol F 1; |
| EINECS: | 204-589-7 |
| Density: | 1.102 g/cm3 |
| Melting Point: | 11-13 °C |
| Boiling Point: | 245.199 °C at 760 mmHg |
| Flash Point: | 105.275 °C |
| Solubility: | Soluble in alcohol, ether and sodium hydroxide solution. Can dissolve oil, natural resin, alkyd, cellulose acetate, ethyecellulose, nitrocellulose and polyvinyl acetate. Miscible with glycerin, propylene glycol, ethanol, ethyl ether, benzene. |
| Appearance: | colorless or light yellow liquid |
| Hazard Symbols: |
 Xn, Xn, Xi Xi
|
| Risk Codes: | 22-36 |
| Safety: | 26 |
| PSA: | 29.46000 |
| LogP: | 1.05770 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

- 122-99-6
2-Phenoxyethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 18h; Ambient temperature; | 100% |
- 22855-36-3
bis(2-phenoxyethyl)carbonate

- 122-99-6
2-Phenoxyethanol

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate Reflux; | 100% |
- 29279-13-8
S-ethyl phenoxythioacetate

- 122-99-6
2-Phenoxyethanol

| Conditions | Yield |
|---|---|
| With tetrabutylammonium borohydride In chloroform for 3h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With [ReOCl2(1,2-bis(diphenylphosphino)ethane)]; hydrogen; potassium tetraphenylborate In tetrahydrofuran at 160℃; under 30003 Torr; for 24h; Autoclave; Inert atmosphere; | 99% |
| With sodium tetrahydroborate; titanium tetrachloride In 1,2-dimethoxyethane for 14h; Ambient temperature; | 95% |
| With strain of the zygomycete fungus S. racemosum MUT 770 In dimethyl sulfoxide for 72h; Enzymatic reaction; | 94% |

| Conditions | Yield |
|---|---|
| With potassium fluoride | 99% |
| With potassium iodide | 97.7% |
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide at 170℃; for 0.583333h; Inert atmosphere; Schlenk technique; | 94% |
| With tetraethylammonium iodide |

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper dichloride at 130℃; for 20h; Inert atmosphere; Schlenk technique; | 99% |

- 122-99-6
2-Phenoxyethanol

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In ethanol for 18h; Debenzylation; Heating; | 98% |
| With indium; ammonium chloride In methanol for 18h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; for 3h; | 98% |
| Stage #1: phenoxyacetic acid ethyl ester With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 4h; Stage #2: With sodium hydroxide In tetrahydrofuran; methanol; water | 88% |
| With lithium aluminium tetrahydride In diethyl ether at 0 - 20℃; for 2h; | 85% |

| Conditions | Yield |
|---|---|
| With strain of the zygomycete fungus S. racemosum MUT 770 In dimethyl sulfoxide for 72h; Enzymatic reaction; | 96% |
| With diisobutylaluminium hydride In toluene at 20℃; for 1.5h; | 73% |
- 143038-69-1
tert-Butyl-dimethyl-(2-phenoxy-ethoxy)-silane

- 122-99-6
2-Phenoxyethanol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol; dichloromethane at 20 - 25℃; for 0.333333h; Flow reactor; | 96% |
| With P(Me2CHNCH2CH2)3N In dimethyl sulfoxide at 80℃; for 36h; desilylation; | 95% |
- 78-67-12,2'-Azobis(2-methylpropionitrile)
- 616202-92-7Lorcaserin
- 7440-44-0Carbon
- 1592-23-0Calcium stearate
- 151767-02-1Montelukast sodium
- 123-86-4Butyl acetate
- 25068-38-6Phenol, 4,4'-(1-methylethylidene)bis-, polymer with (chloromethyl)oxirane
- 65-85-0Benzoic acid
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
2-Phenoxyethanol is an organic compound with the formula C8H10O2, and its systematic name is the same with the product name. With the CAS registry number 122-99-6, it is also named as 2-Hydroxyethyl phenyl ether. It belongs to the product categories of Benzhydrols, Benzyl & Special Alcohols; Ethylene Glycols & Monofunctional Ethylene Glycols; Monofunctional Ethylene Glycols. Its EINECS number is 204-589-7. In addition, the molecular weight is 138.16. Its classification codes are: (1)Anesthetics; (2)Anti-Infective Agents; (3)Anti-infective agents, local; (4)Central Nervous System Agents; (5)Central Nervous System Depressants; (6)Mutation data; (7)Skin / Eye Irritant. This chemical should be sealed and stored in a cool and dry place. It is used as a fixative for perfumes, an insect repellent, a topical antiseptic, a solvent for cellulose acetate, some dyes, inks, and resins, and it is also used in organic synthesis.
Physical properties of 2-Phenoxyethanol are: (1)ACD/LogP: 1.247; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.25; (4)ACD/LogD (pH 7.4): 1.25; (5)ACD/BCF (pH 5.5): 5.22; (6)ACD/BCF (pH 7.4): 5.22; (7)ACD/KOC (pH 5.5): 113.65; (8)ACD/KOC (pH 7.4): 113.65; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 29.46 Å2; (13)Index of Refraction: 1.526; (14)Molar Refractivity: 39.099 cm3; (15)Molar Volume: 127.449 cm3; (16)Polarizability: 15.5×10-24cm3; (17)Surface Tension: 39.97 dyne/cm; (18)Density: 1.084 g/cm3; (19)Flash Point: 105.275 °C; (20)Enthalpy of Vaporization: 50.962 kJ/mol; (21)Boiling Point: 245.199 °C at 760 mmHg; (22)Vapour Pressure: 0.016 mmHg at 25°C.
Preparation: this chemical can be prepared by tert-butyl-dimethyl-(2-phenoxy-ethoxy)-silane at the temperature of 80 °C. This reaction will need reagent P(Me2CHNCH2CH2)3N and solvent dimethylsulfoxide with the reaction time of 36 hours. The yield is about 95%.

Uses of 2-Phenoxyethanol: it can be used to produce N-(2-phenoxyethoxy)phthalimide at the ambient temperature. It will need reagents diethyl azodicarboxylate, triphenylphosphine and solvent tetrahydrofuran with the reaction time of 16 hours. The yield is about 72%.

When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed. It is irritating to eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc(cc1)OCCO
(2)Std. InChI: InChI=1S/C8H10O2/c9-6-7-10-8-4-2-1-3-5-8/h1-5,9H,6-7H2
(3)Std. InChIKey: QCDWFXQBSFUVSP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 490mg/kg (490mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES | Toksikologicheskii Vestnik. Vol. (3), Pg. 37, 1996. |
| mouse | LD50 | oral | 933mg/kg (933mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES | Toksikologicheskii Vestnik. Vol. (3), Pg. 37, 1996. |
| rabbit | LD50 | skin | 5mL/kg (5mL/kg) | Union Carbide Data Sheet. Vol. 6/24/1958, | |
| rat | LD50 | intraperitoneal | 554mg/kg (554mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES | Toksikologicheskii Vestnik. Vol. (3), Pg. 37, 1996. |
| rat | LD50 | oral | 1260mg/kg (1260mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 259, 1941. |
| rat | LD50 | skin | 14422mg/kg (14422mg/kg) | LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | Journal of the American College of Toxicology. Vol. 9, Pg. 259, 1990. |
-
Premium Related Products