Products Categories
| CAS No.: | 123-72-8 |
|---|---|
| Name: | Butyraldehyde |
| Article Data: | 1035 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H8O |
| Molecular Weight: | 72.1069 |
| Synonyms: | Butyraldehyde(8CI);Butal;Butaldehyde;Butanaldehyde;Butyl aldehyde;Butyral;Butyricaldehyde;Butyrylaldehyde;NSC 62779;n-Butanal;n-Butyl aldehyde;n-Butyraldehyde;n-Butyric aldehyde;Butanal; |
| EINECS: | 204-646-6 |
| Density: | 0.784 g/cm3 |
| Melting Point: | -96 °C |
| Boiling Point: | 77.6 °C at 760 mmHg |
| Flash Point: | 12°F |
| Solubility: | water: 7.1 g/100 mL (25 °C) |
| Appearance: | colourless liquid with a very unpleasant smell |
| Hazard Symbols: |
 F F
|
| Risk Codes: | 11 |
| Safety: | 9-29-33 |
| Transport Information: | UN 1129 3/PG 2 |
| PSA: | 17.07000 |
| LogP: | 0.98540 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

| Conditions | Yield |
|---|---|
| With oxidase In water at 40℃; for 1.5h; Reformatsky Reaction; Enzymatic reaction; | 100% |
| With tetramethylammonium monofluorochromate(VI) In dichloromethane at 20℃; for 2h; | 98% |
| With DIQCC In dichloromethane at 20℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| palladium on charcoal In hexane | 100% |
| With sodium tetrahydroborate; nickel dichloride In methanol; water at 20℃; for 0.25h; | 75% |
| With hydrogen; aluminum oxide; titanium-palladium at 100℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; dimethyl sulfoxide at 60℃; for 8h; High pressure; Green chemistry; | 99.9% |
| Stage #1: propyl cyanide With diisobutylaluminium hydride In toluene at -20℃; for 0.222222h; Flow reactor; Stage #2: With water; sodium L-tartrate In toluene at 0℃; chemoselective reaction; | 64% |
| With Diisobutylaluminium hydride(1 M solution in tetrahydrofuran, 4.9 mL, 4.9 mmol) In dichloromethane at -78℃; for 1h; |

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 20℃; for 12h; | 99% |
| With hydrogen; potassium carbonate In acetone at 20℃; under 760.051 Torr; for 0.25h; Concentration; Reagent/catalyst; chemoselective reaction; | 99% |
| With ammonium formate; PdMCM-41 In methanol at 69.84℃; for 1.5h; | 88% |

| Conditions | Yield |
|---|---|
| With tributylphosphine; hydrogen; cobalt(II) acetate In methanol at 85℃; under 63755.1 Torr; for 30h; Irradiation; | 99% |
| With tributylphosphine; hydrogen; cobalt(II) acetate In methanol at 85℃; under 63755.1 Torr; for 30h; Product distribution; Irradiation; other educt and product, different catalysts, temperatures times pressures with and without irradiation; | 99% |
| With hydrogen; 2,7-bis(SO3Na)-4,5-bis(PPh2)-9,9-Me2-xanthene Rh complex at 100℃; under 9075.91 Torr; for 0.00472222h; | 95.6% |
- 21303-03-7
butyric acid Li-salt

- 123-72-8
butyraldehyde

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran for 1h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In methanol; decane at 60℃; for 2.75h; | 96% |
| With zinc dichromate trihydrate at 20℃; grinding; neat (no solvent); chemoselective reaction; | 92% |
| With dipotassium peroxodisulfate; sodium carbonate In water for 0.333333h; Rate constant; Irradiation; pH: 11.5, rate constant (k M-1s-1); |
- 29949-17-5
1,1-diacetoxybutane

- 123-72-8
butyraldehyde

| Conditions | Yield |
|---|---|
| With 2,6-dicarboxypyridinium chlorochromate In acetonitrile at 20℃; for 0.25h; | 95% |
| With N-Bromosuccinimide; water; silica gel at 20℃; for 0.0666667h; neat (no solvent); chemoselective reaction; | 94% |
| With cellulose sulfonic acid In acetonitrile at 50℃; for 0.5h; | 87% |
- 10424-98-3
butanal N,N-dimethylhydrazone

- 123-72-8
butyraldehyde

| Conditions | Yield |
|---|---|
| With iron(II) sulfate In chloroform at 20℃; for 0.75h; Hydrolysis; | 95% |

| Conditions | Yield |
|---|---|
| With (acetylacetonato)dicarbonylrhodium (l); hydrogen In water; toluene at 60℃; under 37503.8 Torr; for 22h; chemoselective reaction; | A 94% B 6% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; hydrogen In water; toluene at 60℃; under 37503.8 Torr; for 22h; chemoselective reaction; |
- 56830-58-12,5-Diamino-4,6-dihydroxypyrimidine hydrochloride
- 144143-96-4Eprosartan mesylate
- 14999-43-0Glucosamine sulfate
- 5419-55-6Boricacid (H3BO3), tris(1-methylethyl) ester
- 63148-57-2Poly(methylhydrosiloxane)
- 507-09-5Thioacetic acid
- 7220-79-3Methylene Blue trihydrate
- 68797-35-3Dipotassium glycyrrhizinate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
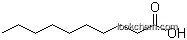
The Butanal, with the CAS registry number 123-72-8 and EINECS registry number 204-646-6, is the aldehyde derivative of butane. It is a colourless flammable liquid with an acrid smell, and miscible with most organic solvents. The molecular formula of this chemical is C4H8O.
The physical properties of Butanal are as followings: (1)ACD/LogP: 0.91; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.91; (4)ACD/LogD (pH 7.4): 0.91; (5)ACD/BCF (pH 5.5): 2.88; (6)ACD/BCF (pH 7.4): 2.88; (7)ACD/KOC (pH 5.5): 74.13; (8)ACD/KOC (pH 7.4): 74.13; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.369; (14)Molar Refractivity: 20.76 cm3; (15)Molar Volume: 91.8 cm3; (16)Polarizability: 8.23×10-24cm3; (17)Surface Tension: 22.5 dyne/cm; (18)Density: 0.784 g/cm3; (19)Enthalpy of Vaporization: 31.86 kJ/mol; (20)Boiling Point: 77.6 °C at 760 mmHg; (21)Vapour Pressure: 96 mmHg at 25°C.
Preparation of Butanal: It can be prepared by the catalytic dehydrogenation of n-butanol. And at one time, it was produced industrially by the catalytic hydrogenation of crotonaldehyde, which is derived from acetaldehyde. It is produced almost exclusively by the hydroformylation of propylene
CH3CH=CH2 + H2 + CO → CH3CH2CH2CHO
Uses of Butanal: It is an important intermediate used in plasticizers, synthetic resins, rubber accelerators and pesticides. It is also a kind of important chemical raw material which is used for the preparation of flavor & aromas.
You should be cautious while dealing with this chemical. It is a kind of highly flammable chemical. Therefore, you had better take the following instructions: Keep container in a well-ventilated place; Do not keep the container sealed; Take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)SMILES: O=CCCC
(2)InChI: InChI=1/C4H8O/c1-2-3-4-5/h4H,2-3H2,1H3
(3)InChIKey: ZTQSAGDEMFDKMZ-UHFFFAOYAZ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LC50 | inhalation | 38gm/m3 (38000mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 51(5), Pg. 61, 1986. | |
| mouse | LC50 | inhalation | 44610mg/m3/2H (44610mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 30, 1982. | |
| mouse | LD50 | intraperitoneal | 1140mg/kg (1140mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 731, 1979. | |
| mouse | LD50 | subcutaneous | 2700mg/kg (2700mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC KIDNEY, URETER, AND BLADDER: HEMATURIA | Acta Pharmacologica et Toxicologica. Vol. 6, Pg. 299, 1950. |
| rabbit | LD50 | skin | 3560uL/kg (3.56mL/kg) | Union Carbide Data Sheet. Vol. 7/20/1965, | |
| rat | LCLo | inhalation | 8000ppm/4H (8000ppm) | National Technical Information Service. Vol. OTS0516688, | |
| rat | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 731, 1979. | |
| rat | LD50 | oral | 2490mg/kg (2490mg/kg) | National Technical Information Service. Vol. OTS0516688, | |
| rat | LD50 | subcutaneous | 10gm/kg (10000mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC | Acta Pharmacologica et Toxicologica. Vol. 6, Pg. 299, 1950. |