Products Categories
| CAS No.: | 153-94-6 |
|---|---|
| Name: | D(+)-Tryptophan |
| Article Data: | 79 |
| Molecular Structure: | |
|
|
|
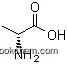
| Formula: | C11H12N2O2 |
| Molecular Weight: | 204.228 |
| Synonyms: | (R)-tryptophan;(+)-Tryptophan;(2R)-2-azaniumyl-3-(1H-indol-3-yl)propanoate;Tryptophan, D-;(2R)-2-amino-3-(1H-indol-3-yl)propanoic acid;H-D-Trp-OH; |
| EINECS: | 205-819-9 |
| Density: | 1.362 g/cm3 |
| Melting Point: | 282-285 °C |
| Boiling Point: | 447.9 °C at 760 mmHg |
| Flash Point: | 224.7 °C |
| Solubility: | 11 g/L (20 °C) in water |
| Appearance: | White or yellow crystalline powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38-41-37/38-22 |
| Safety: | 24/25-36/37/39-36-26 |
| PSA: | 79.11000 |
| LogP: | 1.82260 |
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 211914-50-0N-[[2-[[[4-(Aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-(2-pyridinyl)-beta-alanine ethyl ester hydrochloride

- 153-94-6
D-tryptophan

| Conditions | Yield |
|---|---|
| With benzylamine hydrochloride In water; isopropyl alcohol at 20℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| With CC10H14NOSCH2CH2CH3N(CH3)2 (5); zinc diacetate In methanol at 30℃; Product distribution; pH 4.00; | A 6% B 94% |
| With 5-((3-(dimethylamino)propyl)thio)-4-(aminomethyl)-3-hydroxyl-5,6,7,8-tetraquinoline In methanol at 30℃; Product distribution; stereoselective transaminations at pH=4.00, various reagents; | A 6 % Chromat. B 94 % Chromat. |
| With phosphate buffer; 6A-monoimidazolyl-6B-(5'-thiopyridoxaminyl)-β-D-cyclodextrin In water at 25℃; Product distribution; other reragents, various pH; |

| Conditions | Yield |
|---|---|
| Stage #1: indole; L-serin With Salmonella enterica tryptophan synthase In methanol; aq. phosphate buffer at 37℃; pH=8.0; Enzymatic reaction; Stage #2: With (2R)-aspartic acid In methanol; aq. phosphate buffer pH=8.0; Stage #3: In aq. phosphate buffer; deuteromethanol at 37℃; pH=8.0; Enzymatic reaction; stereoselective reaction; | 69% |
- 66872-76-2
β-(3-indolyl)-α-ketopropanoic acid sodium salt

- 153-94-6
D-tryptophan

| Conditions | Yield |
|---|---|
| With zinc(II) perchlorate; (S)-15-amino-methyl-14-hydroxy-5,5-dimethyl-2,8-dithia<9>(2,5)pyridinophane In methanol for 24h; Ambient temperature; | 62% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; oxygen; 2-amino-2-hydroxymethyl-1,3-propanediol In water at 30℃; for 8h; pH=7.3; Resolution of racemate; Enzymatic reaction; enantioselective reaction; | 48% |

| Conditions | Yield |
|---|---|
| With Bis(2-ethylhexyl)phosphoric acid; sodium dodecyl-sulfate; O,O'-dibenzoyl-L-tartaric acid In octanol; water at 20℃; for 4h; Reflux; Resolution of racemate; optical yield given as %ee; enantioselective reaction; | A n/a B 18.59% |
| With (2R,3R,11R,12R)-(+)-18-crown-6-2,3,11,12-tetracarbonic acid In acetonitrile at 25℃; pH=3; Electrochemical reaction; | |
| With teicoplanin In methanol; water Product distribution; Further Variations:; Reagents; pH-values; Solvents; |
- 4299-70-1, 7303-49-3, 22032-65-1
D-Tryptophan methyl ester

- 153-94-6
D-tryptophan

| Conditions | Yield |
|---|---|
| With barium dihydroxide; water | |
| With methanol; barium dihydroxide | |
| With Streptomyces spp. 82F2 D-aminopeptidase In dimethyl sulfoxide at 20℃; for 0.0833333h; pH=6.5; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water | |
| With sulfuric acid; water | |
| With barium dihydroxide; water | |
| With D-aminoacylase; cobalt(II) chloride hexahydrate at 36 - 37℃; pH=7.4; aq. phosphate buffer; Enzymatic reaction; |
- 742100-62-5
Nα-chloroacetyl-D-tryptophan

- 153-94-6
D-tryptophan

| Conditions | Yield |
|---|---|
| With sulfuric acid; water |

| Conditions | Yield |
|---|---|
| With methanol; palladium; acetic acid Hydrogenation; |
- 93106-60-6Enrofloxacin
- 2216-51-5Cyclohexanol,5-methyl-2-(1-methylethyl)-, (1R,2S,5R)-
- 128-08-5N-Bromosuccinimide
- 87-51-41H-Indole-3-acetic acid
- 58-93-5Hydrochlorothiazide
- 64485-93-4Cefotaxime sodium
- 54060-30-93-Aminophenylacetylene
- 54965-24-1Tamoxifen citrate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
Reported in EPA TSCA Inventory.
Specification
The IUPAC name of D-Tryptophan is 2-amino-3-(1H-indol-3-yl)propanoic acid. With the CAS registry number 153-94-6, it is also named as (R)-a-Amino-3-indolepropionic Acid. The product's categories are Amino Acids Derivatives; Tryptophan [Trp, W]; Amino Acids and Derivatives; alpha-Amino Acids; Amino Acids; Biochemistry; Indoles; Tryptophans; Chiral Compound; Amino Acids. It is white or yellow crystalline powder which is soluble in hot ethanol, alkali solution and water, insoluble in chloroform. In addition, this chemical is combustible, stable and incompatible with oxidizing agents. Besides, it should be sealed in the container and stored in the cool and dry place.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 1.04; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.46; (4)ACD/LogD (pH 7.4): -1.46; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 4; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 4; (12)Index of Refraction: 1.697; (13)Molar Refractivity: 57.76 cm3; (14)Molar Volume: 149.8 cm3; (15)Polarizability: 22.9×10-24 cm3; (16)Surface Tension: 71.1 dyne/cm; (17)Enthalpy of Vaporization: 74.44 kJ/mol; (18)Vapour Pressure: 8.3E-09 mmHg at 25°C; (19)Rotatable Bond Count: 3; (20)Exact Mass: 204.089878; (21)MonoIsotopic Mass: 204.089878; (22)Topological Polar Surface Area: 79.1; (23)Heavy Atom Count: 15; (24)Complexity: 245.
Preparation of D-Tryptophan: Using DL-tryptophan as raw materials, and adding chloroacetic anhydride to react in cooling condition. Grinding in ice water after sulfuric acid acidizing. Then filtering and recrystallizing in water. Finally, treating with pancreatic carboxypeptidase to remove the L-type. Then we can get the product by acetic acid acidification and ethanol recrystallization.
Uses of of D-Tryptophan: It can used in biochemistry research. And it is an important nutrient which is used as controlling agent of bark favus in medication.
When you are using this chemical, please be cautious about it as the following:
It is not only harmful if swallowed, but also irritating to eyes, respiratory system and skin. So people should avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection.
People can use the following data to convert to the molecule structure.
1. SMILES:O=C(O)[C@H](N)Cc2c1ccccc1nc2
2. InChI:InChI=1/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m1/s1
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 4289mg/kg (4289mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Archives of Biochemistry and Biophysics. Vol. 64, Pg. 319, 1956. |
-
Premium Related Products