Products Categories
| CAS No.: | 353-50-4 |
|---|---|
| Name: | CARBONYL FLUORIDE |
| Article Data: | 486 |
| Molecular Structure: | |
|
|
|
| Formula: | CF2 O |
| Molecular Weight: | 66.0072 |
| Synonyms: | Carbonylfluoride (6CI,8CI); Carbon difluoride oxide; Carbon fluoride oxide (COF2);Carbon oxyfluoride; Carbon oxyfluoride (COF2); Carbonyl difluoride; Carbonyldifluoride (COF2); Carbonyl fluoride (COF2); Difluoroformaldehyde;Difluorooxomethane; Difluorophosgene; Fluoroformyl fluoride; Fluorophosgene |
| Density: | 1.244g/cm3 |
| Melting Point: | -114°C |
| Boiling Point: | -84°C |
| Flash Point: | °C |
| Solubility: | instantly hydrolyzed by H2O [MER06] |
| Hazard Symbols: | Toxic by inhalation, strong irritant to skin. TLV: 2 ppm. |
| Risk Codes: | 8-23/24/25-34 |
| Safety: | A poison. Moderately toxic by inhalation. A powerful irritant. Hydrolyzes instantly to form HF on contact with moisture. See also CARBONYLS, HYDROFLUORIC ACID, and FLUORINE. Incompatible with hexafluoroisopropylideneamino-lithium. When heated to decomposition it emits toxic fumes of CO and F−. See CARBON MONOXIDE for fire and explosion hazard. |
| PSA: | 17.07000 |
| LogP: | 1.04540 |
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- Total:2 Page 1 of 1 1
Chemistry
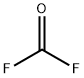
Molecular Structure of Carbonyl fluoride (CAS NO. 353-50-4):
EINECS: 206-534-2
IUPAC Name: Carbonyl difluoride
Molecular Formula: CF2O
Molecular Weight: 66.006906 g/mol
XLogP3-AA: 0.8
H-Bond Donor: 0
H-Bond Acceptor: 3
Canonical SMILES: C(=O)(F)F
InChI: InChI=1S/CF2O/c2-1(3)4
InChIKey: IYRWEQXVUNLMAY-UHFFFAOYSA-N
Index of Refraction: 1.207
Molar Refractivity: 7.03 cm3
Molar Volume: 53 cm3
Surface Tension: 10.7 dyne/cm
Density: 1.244 g/cm3
Enthalpy of Vaporization: 17.99 kJ/mol
Boiling Point: -84 °C
Vapour Pressure: 29200 mmHg at 25 °C
Melting Point: -114 °C
Water Solubility: 1e+006 mg/L at 25 °C
Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LC50 | inhalation | 360ppm/1H (360ppm) | LUNGS, THORAX, OR RESPIRATION: CONSOLIDATION LUNGS, THORAX, OR RESPIRATION: FIBROSIS (INTERSTITIAL) LIVER: FATTY LIVER DEGERATION | American Industrial Hygiene Association Journal. Vol. 29, Pg. 41, 1968. |
Consensus Reports
Reported in EPA TSCA Inventory.
Safety Profile
Safety Information of Carbonyl fluoride (CAS NO.353-50-4):
Hazard Codes: T 
Hazard Note: Highly Toxic
Risk Statements: 8-23/24/25-34
R8 :Contact with combustible material may cause fire
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed
R34:Causes burns
Safety Statements: 3/7-9-36/37/39-38-45
S3:Keep in a cool place
S7:Keep container tightly closed
S9:Keep container in a well-ventilated place
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S38:In case of insufficient ventilation, wear suitable respiratory equipment
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: 2417
HazardClass: 2.3
A poison. Moderately toxic by inhalation. A powerful irritant. Hydrolyzes instantly to form HF on contact with moisture. See also CARBONYLS, HYDROFLUORIC ACID, and FLUORINE. Incompatible with hexafluoroisopropylideneamino-lithium. When heated to decomposition it emits toxic fumes of CO and F−. See CARBON MONOXIDE for fire and explosion hazard.
Standards and Recommendations
OSHA PEL: TWA 2 ppm; STEL 5 ppm
ACGIH TLV: TWA 2 ppm; STEL 5 ppm
DOT Classification: 2.3; Label: Poison Gas
Specification
Carbonyl fluoride with cas registry number of 353-50-4 is pungent colorless gas. It is also called for Carbon difluoride oxide ; Carbon fluoride oxide (COF2) ; Carbon oxyfluoride (COF2) ; Carbon oxyfluoride ; Carbonic difluoride ; Carbonyl difluoride ; Carbonyl oxyfluoride ; Difluoroformaldehyde ; Difluorooxomethane ; Fluophosgene ; Fluoroformyl fluoride ; Fluorophosgene ; HSDB 6010 ; RCRA waste number U033 . It is unstable in the presence of water and incompatible with water , bases (including amines), oxidizing agents, alcohols. When heated to decomposition, it yields carbon monoxide gas and fluorine . It may react vigorously if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts and reacts violently with hexafluoroisopropylideneaminolithium. It can be used for the production of fluorine plastics.

