Products Categories
| CAS No.: | 37793-53-6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name: | N10-(TRIFLUOROACETYL)PTEROIC ACID | ||||||||
| Article Data: | 16 | ||||||||
| Molecular Structure: | |||||||||
|
|
|||||||||
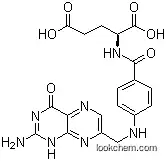
| Formula: | C16H11 F3 N6 O4 | ||||||||
| Molecular Weight: | 408.296 | ||||||||
| Synonyms: | Benzoicacid, 4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl](trifluoroacetyl)amino]-(9CI);N10-(Trifluoroacetyl)pteroic acid;N10-Trifluoroacetylpteroic acid; | ||||||||
| Density: | 1.74g/cm3 | ||||||||
| Melting Point: |
270 °C (dec.)(lit.) |
||||||||
| Boiling Point: | 650.2°Cat760mmHg | ||||||||
| Flash Point: | 347.1°C | ||||||||
| Hazard Symbols: |

|
||||||||
| Risk Codes: | 45-20/21/22-36/37/38 | ||||||||
| Safety: |
|
||||||||
| PSA: | 155.42000 | ||||||||
| LogP: | 2.08250 | ||||||||
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 43224-75-52,2,6,6-Tetramethyl-4-(2-propyleneoxy) Piperidine
- 223378-68-5
N10-(trifluoroacetyl)pyrofolic acid

A
- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride Hydrolysis; | A 56% B 33% |
- 59-30-3
folic acid

- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tetrahydrofuran / 10.5 h / 0 - 25 °C 1.2: 123 g / ice / tetrahydrofuran / 3 h / 25 °C 2.1: 56 percent / aq. HCl View Scheme |
- 197151-79-4
pteroyl azide

- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 67 percent / tetramethylguanidine / dimethylsulfoxide / 9 h / 25 °C 2.1: tetrahydrofuran / 10.5 h / 0 - 25 °C 2.2: 123 g / ice / tetrahydrofuran / 3 h / 25 °C 3.1: 56 percent / aq. HCl View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: pteroic acid; trifluoroacetic anhydride at 20℃; for 96h; Stage #2: With trifluoroacetic acid In water for 48.3333h; | |
| at 20℃; for 24h; Inert atmosphere; Darkness; | |
| With trifluoroacetic acid | |
| at 20℃; for 24h; Inert atmosphere; Darkness; |
- 119-24-4
pteroic acid

- 76-05-1
trifluoroacetic acid

- 407-25-0
trifluoroacetic anhydride

- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| Stage #1: pteroic acid; trifluoroacetic anhydride at 20℃; for 96h; Sealed tube; Inert atmosphere; Stage #2: trifluoroacetic acid at 20℃; for 48h; Sealed tube; Inert atmosphere; |

- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 3h; | 100% |
- 37793-53-6
N10-trifluoroacetylpteroic acid

- 6234-01-1
L-glutamic acid 5-tert-butyl 1-methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: N10-trifluoroacetylpteroic acid; L-glutamic acid 5-tert-butyl 1-methyl ester hydrochloride With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 23℃; for 0.25h; Inert atmosphere; Stage #2: With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate In dimethyl sulfoxide at 23℃; for 24h; Inert atmosphere; | 100% |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 23℃; Inert atmosphere; |
- 37793-53-6
N10-trifluoroacetylpteroic acid

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Inert atmosphere; | 100% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Inert atmosphere; | 4.06 g |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Inert atmosphere; | 4.06 g |
- 37793-53-6
N10-trifluoroacetylpteroic acid

- 17083-23-7
2-amino-3-(4-tert-butoxy-phenyl)-propionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 23℃; for 2.16667h; Inert atmosphere; | 96.7% |
- 37793-53-6
N10-trifluoroacetylpteroic acid

- 1375540-44-5
(S)-tert-butyl 2-amino-5-((3-azidopropyl)amino)-5-oxopentanoate

- 1375540-45-6
(S)-tert-butyl 2-(4-(N-((2-amino-4-oxo-3,4-dihydropteridin-6-yl)methyl)-2,2,2-trifluoroacetamido)benzamido)-5-((3-azidopropyl)amino)-5-oxopentanoate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; for 48h; | 52% |
- 301-04-2Acetic acid, lead(2+)salt (2:1)
- 629-82-3Dioctyl ether
- 589-08-2Benzeneethanamine,N-methyl-
- 68424-85-1Benzalkonium chloride
- 8002-75-3Palm oil
- 1344-00-9Silicic acid, aluminum sodium salt
- 13825-74-6Titanium,oxo[sulfato(2-)-kO,kO']-
- 81025-04-9D-Glucitol, 4-O-b-D-galactopyranosyl-, hydrate(1:1)
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Specification
The N10-(Trifluoroacetyl)pteroic acid, with the CAS registry number 37793-53-6, is also known 4-[(2-Amino-4-hydroxypteridin-6-ylmethyl)-(2,2,2-trifluoroacetyl)amino]benzoic acid.It belongs to the product Organic matters.This chemical's molecular formula is C16H11F3N6O4 and molecular weight is 408.2. What's more,Its systematic name is 4-{[(2-Amino-4-oxo-1,4-dihydro-6-pteridinyl)methyl](trifluoroacetyl)amino}benzoic acid.
Physical properties about N10-(Trifluoroacetyl)pteroic acid are: (1)ACD/LogP: 0.44; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): -1.04; (4)ACD/LogD (pH 7.4): -2.51; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.36; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 10; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 4; (12)Index of Refraction: 1.698; (13)Molar Refractivity: 90.276 cm3; (14)Molar Volume: 234.096 cm3; (15)Surface Tension: 65.5690002441406 dyne/cm; (16)Density: 1.744 g/cm3; (17)Flash Point: 347.051 °C; (18)Enthalpy of Vaporization: 100.711 kJ/mol; (19)Boiling Point: 650.237 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25°C.
You can still convert the following datas into molecular structure:
(1)SMILES:FC(F)(F)C(=O)N(c1ccc(C(=O)O)cc1)Cc2nc3c(nc2)N/C(=N\C3=O)N;
(2)Std. InChI:InChI=1S/C16H11F3N6O4/c17-16(18,19)14(29)25(9-3-1-7(2-4-9)13(27)28)6-8-5-21-11-10(22-8)12(26)24-15(20)23-11/h1-5H,6H2,(H,27,28)(H3,20,21,23,24,26);
(3)Std. InChIKey:IJGIHDXKYQLIMA-UHFFFAOYSA-N;