Products Categories
| CAS No.: | 491-70-3 |
|---|---|
| Name: | Luteolin |
| Article Data: | 100 |
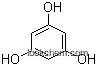
| Molecular Structure: | |
|
|
|
| Formula: | C15H10O6 |
| Molecular Weight: | 286.241 |
| Synonyms: | 4H-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-;Cyanidenon 1470;Prestwick_122;Flacitran;4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy- (9CI);2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-benzopyrone;2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one;4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-;3,4,5,7-Tetrahydroxyflavone;5,7,3,4-Tetrahydroxyflavone;C.I. Natural Yellow 2;2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-chromen-4-one;C.I. 75590;Luteolol;Digitoflavone;3',4',5,7-Tetrahydroxyflavone; |
| EINECS: | 207-741-0 |
| Density: | 1.654 g/cm3 |
| Melting Point: | ~330 °C(lit.) |
| Boiling Point: | 616.1 °C at 760 mmHg |
| Flash Point: | 239.5 °C |
| Solubility: | Soluble in aqueous alkaline solutions (1.4 mg/ml), ethanol (~5 mg/ml), dimethyl sulfoxide (7 mg/ml), 1eq. Sodium hydroxide (5 mM), dimethylformamide (~20 mg/ml), water (1 mg/ml) at 25°C and methanol. |
| Appearance: | yellow crystalline powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 26-36-36/37/39 |
| PSA: | 111.13000 |
| LogP: | 2.28240 |
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 520-36-5
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With C9H8IO4Pol In dimethyl sulfoxide at 25℃; for 2h; | 95% |
| With cytochromes P450 in human liver microsomes Kinetics; Enzymatic reaction; |
- 855-97-0
2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-4H-chromen-4-one

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With pyridine hydrochloride at 190℃; for 6h; Inert atmosphere; | 89% |
| With pyridine hydrochloride at 180℃; for 6.5h; Inert atmosphere; | 88% |
| Stage #1: 2-(3,4-dimethoxyphenyl)-5,7-dimethoxy-4H-chromen-4-one With aluminum (III) chloride In toluene at 80 - 140℃; Stage #2: With hydrogenchloride In water; toluene at 0℃; Reagent/catalyst; Solvent; Temperature; Time; | 70.2% |
- 58124-13-3
2-(3,4-bis(benzyloxy)phenyl)-5,7-dihydroxy-4H-chromen-4-one

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In N,N-dimethyl-formamide Ambient temperature; | 80% |

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid at 95 - 100℃; for 1h; | 78% |
- 14917-41-0
2',3,4,4',6'-pentahydroxychalcone

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With oxygen at 20℃; under 760.051 Torr; for 1h; Reagent/catalyst; | 72% |
- 5373-11-5, 68321-11-9
luteolin 7-O-glucoside

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With sulfuric acid for 4h; | 68.2% |
| With acid | |
| With sulfuric acid; water for 2h; Heating; | 70 mg |
- 5373-11-5, 68321-11-9
luteolin 7-O-glucoside

A
- 50-99-7
D-glucose

B
- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With water Acidic conditions; | A n/a B 66% |
| Acid hydrolysis; | |
| Acidic aq. solution; | |
| Acidic conditions; |

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-bis(methoxymethyl)-2-(3,4-diacetoxybenzoyloxy)acetophenone With potassium hydroxide In pyridine at 50℃; for 0.333333h; Stage #2: With acetic acid In pyridine; water for 0.5h; Stage #3: With hydrogenchloride In methanol Reflux; | 55% |
- 3563-98-2, 20633-84-5
scolymoside

- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With sulfuric acid; water for 2h; Heating; | 50% |
- 480-40-0
5,7-dihydroxy-2-phenyl-chromen-4-one

A
- 520-36-5
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one

B
- 491-70-3
2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-on

| Conditions | Yield |
|---|---|
| With Mucore ramannianus (ATCC 9628) In N,N-dimethyl-formamide for 336h; Microbiological reaction; | A 3% B 10.2% C 10.2% |

- 5593-20-4Betamethasone 17,21-dipropionate
- 113665-84-2Clopidogrel
- 109-04-62-Bromopyridine
- 80474-14-2Fluticasone propionate
- 10238-21-8Benzamide,5-chloro-N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-2-methoxy-
- 2398-37-03-Bromoanisole
- 84650-60-2Tea polyphenol
- 29385-43-1Tolyltriazole
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Chemistry
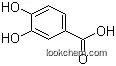
Molecular Structure of Luteolin (CAS NO.491-70-3):
.png)
IUPAC Name: 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one
Empirical Formula: C15H10O6
Molecular Weight: 286.2363
H bond acceptors: 6
H bond donors: 4
Freely Rotating Bonds: 5
Polar Surface Area: 63.22Å2
Index of Refraction: 1.767
Molar Refractivity: 71.73 cm3
Molar Volume: 172.9 cm3
Surface Tension: 92.5 dyne/cm
Density: 1.654 g/cm3
Flash Point: 239.5 °C
Enthalpy of Vaporization: 94.73 kJ/mol
Boiling Point: 616.1 °C at 760 mmHg
Vapour Pressure: 9.03E-16 mmHg at 25°C
Melting point: 330 oC
Storage temp: 2-8oC
EINECS: 207-741-0
Product Categories: Tetra-substituted Flavones; Natural Plant Extract; Inhibitors; Tyrosine Kinase Inhibitors; Cytokine signaling
InChI
InChI=1/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
Smiles
c12c(c(cc(o1)c1cc(c(O)cc1)O)=O)c(cc(c2)O)O
Uses
Luteolin (CAS NO.491-70-3) is thought to play an important role in the human body as an antioxidant, a free radical scavenger, an agent in the prevention of inflammation, a promoter of carbohydrate metabolism and an immune system modulator. Multiple research experiments describe luteolin as a biochemical agent that can dramatically reduce inflammation and the symptoms of septic shock.These characteristics of luteolin are also believed to play an important part in the prevention of cancer.
Toxicity Data With Reference
| 1. | mnt-hmn-lym 10 mg/L | MUREAV Mutation Research. 246 (1991),205. | ||
| 2. | sce-hmn-lym 10 mg/L | MUREAV Mutation Research. 246 (1991),205. | ||
| 3. | sln-hmn-lym 20 mg/L | MUREAV Mutation Research. 246 (1991),205. | ||
| 4. | ipr-mus LD50:180 mg/kg | YHTPAD Yaoxue Tongbao. Bulletin of Pharmacology. 16 (2)(1981),11. |
Safety Profile
A poison by intraperitoneal route. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 3
RTECS: LK9275210
Specification
Luteolin , with CAS number of 491-70-3, can be called 3',4',5,7-Tetrahydroxyflavone ; 5,7,3',4'-Tetrahydroxyflavone ; Digitoflavone ; Luteoline ; Salifazide . Luteolin (CAS NO.491-70-3) is often found in leaves, but it is also seen in celery, thyme, dandelion, rinds, barks, clover blossom and ragweed pollen. Luteolin has also been isolated from Salvia tomentosa. Dietary sources include celery, green pepper, thyme, perilla and camomile tea.