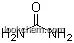Products Categories
| CAS No.: | 50-06-6 |
|---|---|
| Name: | Phenobarbital |
| Article Data: | 33 |
| Molecular Structure: | |
|
|
|
| Formula: | C12H12N2O3 |
| Molecular Weight: | 232.239 |
| Synonyms: | Barbituricacid, 5-ethyl-5-phenyl- (8CI);5-Ethyl-5-phenylbarbituric acid;5-Phenyl-5-ethylbarbituric acid;Adonal;Agrypnal;Amylofene;Barbenyl;Barbiphenyl;Barbipil;Barbita;Barbivis;Blu-phen;Cratecil;Dormiral;Doscalun;Duneryl;Eskabarb;Etilfen;Euneryl;Fenemal;Fenemal recip;Gardenal;Gardepanyl;Hysteps;Lepinal;Lepinaletten;Liquital;Lixophen;Lubergal;Luminal;NSC 128143;NSC 9848;Neurobarb;Noptil;Nunol;Phenaemal;Phenemal;Phenobar;Phenobarbital;Phenobarbitone;Phenobarbituric acid;Phenoluric;Phenonyl;Phenylethylbarbituric acid;Phenylethylmalonylurea;Phenyral;Phob;Sedonal;Sedophen;Sevenal;Solfoton;Somonal;StentalExtentabs;Talpheno;Teolaxin;Triphenatol;Versomnal;A0038 Cyclic-peoxide-containing acid 3; |
| EINECS: | 200-007-0 |
| Density: | 1.234 g/cm3 |
| Melting Point: | 174 °C |
| Boiling Point: | 374.4°C (rough estimate) |
| Flash Point: | 11 °C |
| Solubility: | < 0.01 g/100 mL at 14 °C in water |
| Appearance: | Crystalline Solid |
| Hazard Symbols: |
 T T
|
| Risk Codes: | 25-40-43-61 |
| Safety: | 53-36/37-45 |
| Transport Information: | UN 2811 |
| PSA: | 75.27000 |
| LogP: | 1.35800 |
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
History
The first barbiturate drug, barbital, was synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering at Bayer. By 1904 several related drugs, including Phenobarbital (CAS NO.50-06-6),had been synthesized by Fischer. Phenobarbital (CAS NO.50-06-6) was brought to market in 1912 by the drug company Bayer using the brand Luminal. It remained a commonly prescribed sedative and hypnotic until the introduction of benzodiazepines in the 1950s.Phenobarbital's soporific, sedative and hypnotic properties were well known in 1912, but nobody knew it was also an effective anticonvulsant.
Between 1934-1945 Phenobarbital (CAS NO.50-06-6) under the brand name Luminal, was used by German doctors under the Nazi party endorsed policy of eugenics to remove children born with disease or deformities from the population so that they would not suffer. Many of the medical staff involved were later to transfer to Nazi hospitals. Phenobarbital (CAS NO.50-06-6) was used to treat neonatal jaundice by increasing liver metabolism and thus lowering bilirubin levels. In the 1950s, phototherapy was discovered, and became the standard treatment.
Consensus Reports
EPA Genetic Toxicology Program. IARC Cancer Review: Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 313.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 313.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 13 (1977),p. 157.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) .
Specification
The Phenobarbital, with the CAS registry number 50-06-6 and EINECS registry number 200-007-0, has the IUPAC name of 5-ethyl-5-phenyl-1,3-diazinane-2,4,6-trione. It is a kind of crystalline solid, and belongs to the following proudct categories: Aromatics; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals. And the molecular formula of this chemical is C12H12N2O3. What's more, it should be stored at 2-8°C.
The physical properties of Phenobarbital are as followings: (1)ACD/LogP: 1.67; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.67; (4)ACD/LogD (pH 7.4): 1.47; (5)ACD/BCF (pH 5.5): 10.83; (6)ACD/BCF (pH 7.4): 6.93; (7)ACD/KOC (pH 5.5): 191.18; (8)ACD/KOC (pH 7.4): 122.28; (9)#H bond acceptors: 5; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 57.69 Å2; (13)Index of Refraction: 1.541; (14)Molar Refractivity: 59.21 cm3; (15)Molar Volume: 188.1 cm3; (16)Polarizability: 23.47×10-24cm3; (17)Surface Tension: 43.3 dyne/cm; (18)Density: 1.234 g/cm3.
Preparation of Phenobarbital: It can start with the condensation of benzyl cyanide with diethylcarbonate in the presence of sodium ethoxide, and the result is α-phenylcyanoacetic ester. Alkylation of the ester needs ethyl bromide, and gives α-phenyl-α-ethylcyanoacetic ester, which is further converted into the 4-iminoderivative upon treatment with urea. And acidic hydrolysis of the resulting product gives phenobarbital.

Uses of Phenobarbital: It is widely used as a anticonvulsant . And it is often used for the treatment of acute withdrawal from benzodiazepines. What's more, it also inhibits glutamate induced depolarizations.
You should be cautious while dealing with this chemical. It is toxic if swallowed, and may cause sensitization by skin contact. It has risk of serious damage to eyes, and it may also cause harm to the unborn child. Therefore, you had better take the following instructions: Avoid exposure - obtain special instruction before use. Wear suitable protective clothing and gloves, and in case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1NC(=O)NC(=O)C1(c2ccccc2)CC
(2)InChI: InChI=1/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17)
(3)InChIKey: DDBREPKUVSBGFI-UHFFFAOYAB
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | oral | 125mg/kg (125mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| cat | LDLo | subcutaneous | 125mg/kg (125mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| child | TDLo | oral | 10mg/kg (10mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. |
| child | TDLo | oral | 20mg/kg/I (20mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ATAXIA BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Clinical Pediatrics Vol. 31, Pg. 252, 1992. |
| dog | LD50 | oral | 150mg/kg (150mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Schweizerische Medizinische Wochenschrift. Vol. 85, Pg. 305, 1955. |
| dog | LDLo | subcutaneous | 150mg/kg (150mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| frog | LDLo | subcutaneous | 500mg/kg (500mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| guinea pig | LD50 | oral | 130mg/kg (130mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 91, Pg. 437, 1952. | |
| human | TDLo | oral | 18mg/kg (18mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | JAMA, Journal of the American Medical Association. Vol. 116, Pg. 700, 1941. |
| infant | TDLo | intramuscular | 50mg/kg (50mg/kg) | BEHAVIORAL: COMA | Pediatrics. Vol. 77, Pg. 848, 1986. |
| man | LDLo | oral | 6485ug/kg/2W- (6.485mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | Annals of Internal Medicine. Vol. 13, Pg. 1243, 1940. |
| mouse | LD50 | intramuscular | 175mg/kg (175mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 2215, 1981. | |
| mouse | LD50 | intraperitoneal | 88mg/kg (88mg/kg) | Pharmazie. Vol. 52, Pg. 926, 1997. | |
| mouse | LD50 | intravenous | 218mg/kg (218mg/kg) | PERIPHERAL NERVE AND SENSATION: LOCAL ANESTHETIC | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 191, Pg. 405, 1971. |
| mouse | LD50 | oral | 137mg/kg (137mg/kg) | Pharmaceutical Chemistry Journal Vol. 10, Pg. 41, 1976. | |
| mouse | LD50 | subcutaneous | 228mg/kg (228mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 30, Pg. 477, 1980. | |
| rabbit | LD50 | intravenous | 187mg/kg (187mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 1168, 1983. |
| rabbit | LD50 | oral | 185mg/kg (185mg/kg) | Neuropatologia Polska. Vol. 15, Pg. 545, 1977. | |
| rabbit | LDLo | intraperitoneal | 150mg/kg (150mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 44, Pg. 325, 1932. | |
| rabbit | LDLo | subcutaneous | 150mg/kg (150mg/kg) | Journal of the American Chemical Society. Vol. 45, Pg. 243, 1923. | |
| rat | LC | inhalation | > 4100ug/m3/4H (4.1mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: OTHER CHANGES | Toksikologicheskii Vestnik. Vol. (1), Pg. 39, 2000. |
| rat | LD50 | intraperitoneal | 110mg/kg (110mg/kg) | French Medicament Patent Document. Vol. #7157M, | |
| rat | LD50 | intravenous | 209mg/kg (209mg/kg) | Archives of Toxicology. Vol. 40, Pg. 211, 1978. | |
| rat | LD50 | oral | 162mg/kg (162mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| rat | LD50 | rectal | 284mg/kg (284mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: GENERAL ANESTHETIC | Anesthesia and Analgesia Vol. 46, Pg. 395, 1967. |
| rat | LD50 | subcutaneous | 200mg/kg (200mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 152, Pg. 341, 1930. | |
| women | LDLo | oral | 25272ug/kg/13 (25.272mg/kg) | BEHAVIORAL: COMA SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | Canadian Medical Association Journal. Vol. 33, Pg. 635, 1935. |
| women | TDLo | oral | 46mg/kg (46mg/kg) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Clinical Pediatrics Vol. 24, Pg. 678, 1985. |