1538-09-6 Usage
Description
Benzathine benzylpenicillin, also known as benzathine penicillin, is a penicillin-class antibiotic that is used to combat bacterial infections. It is a white or almost white powder and is administered through intramuscular injection. The mechanism of action involves inhibiting the cell wall synthesis of bacteria, leading to their destruction.
Uses
Used in Pharmaceutical Industry:
Benzathine benzylpenicillin is used as an antibiotic for the treatment of various bacterial infections. It is particularly effective against infections caused by Streptococcus, Staphylococcus aureus, and other susceptible bacteria. The application reason is its ability to inhibit bacterial cell wall synthesis, effectively killing the bacteria and treating the infection.
Used in Preventive Medicine:
Benzathine benzylpenicillin is used as a preventive measure for rheumatic fever. The application reason is its effectiveness in treating or preventing the recurrence of streptococcal infections, which are known to cause rheumatic fever.
Used in Combination Therapy:
Benzathine benzylpenicillin is also used in combination with other antibiotics or medications to enhance the treatment of more severe or resistant infections. The application reason is its synergistic effect when combined with other drugs, which can improve the overall efficacy of the treatment and help overcome bacterial resistance.
Originator
Bicillin,Wyeth,US,1951
Manufacturing Process
Ethylenediamine (15 g, 0.25 mol) was added dropwise to 100 ml 98-100% formic acid in a two-necked 500 ml flask, fitted with an addition tube and reflux condenser with drying tube, cooled in an ice-bath. After complete addition of the base, 53 g of benzaldehyde (0.5 mol) was added in one lot. The ice-bath was removed and the flask was heated to the refluxing temperature. The initial rate of carbon dioxide evolution was too rapid to measure. After twenty minutes, the rate was circa 100 ml per minute and decreased rapidly to 8 ml per minute in one hour. Heating at reflux was continued for 35 hours.
Following the refluxing most of the excess formic acid was removed under reduced pressure. Hydrochloric acid (200 ml 6 N) was added to the viscous amber residue and heated under reflux, After 15 minutes, bumping necessitated cooling and filtering to remove crystalline dihydrochloride, which after washing with isopropanol was dried, MP circa 300°C. The mother liquors were refluxed one hour and cooled, obtaining an additional amount of product, MP circa 300°C. The filtrate was concentrated in vacuo to 100 ml, cooled and made alkaline with 40% NaOH. The supernatant oil was extracted with ether, dried, and fractionated from a stillpot packed with glass wool and heated in a sand-bath at 320°C. The first fraction at 106°C at 0.6-0.7 mm was Nbenzylethylenediamine (dipicrate, MP 222°C). The N,N'dibenzylethylenediamine was collected at 177°C to 206°C at 0.6-1.0 mm as a colorless liquid.
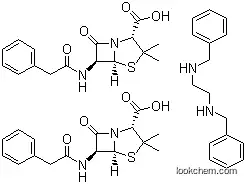
To a solution of 60 g of sodium penicillin G in 800 cc of distilled water cooled to 0°C to 4°C in an ice-bath, a solution of 35 g of N,N'dibenzylethylenediamine diacetate in 200 cc of distilled water is added dropwise with stirring. The thick slurry is filtered with suction, washed twice with 100 cc of cold water, dried by suction and spread out in a thin layer for completion of drying. The product weighed 80 g.
The air-dried powder has a broad melting point, sintering at 100°C, melting above 110°C to a cloudy liquid becoming clear at 135°C.
Therapeutic Function
Antibacterial
Clinical Use
Since penicillin G benzathine, N,N'-dibenzylethylenediaminedipenicillin G (Bicillin, Permapen), is the salt of a diamine,2 moles of penicillin are available from each molecule. It isvery insoluble in water, requiring about 3,000 mL to dissolve1 g. This property gives the compound great stabilityand prolonged duration of effect. At the pH of gastric juice,it is quite stable, and food intake does not interfere with itsabsorption. It is available in tablet form and in several parenteralpreparations. The activity of penicillin G benzathineis equivalent to 1,211 units/mg.Several other amines have been used to make penicillinsalts, and research is continuing on this subject. Otheramines that have been used include 2-chloroprocaine; L-Nmethyl-1,2-diphenyl-2-hydroxyethylamine (L-ephenamine);dibenzylamine; tripelennamine (Pyribenzamine); and N,N'-bis-(dehydroabietyl)ethylenediamine (hydrabamine).
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. Experimental reproductive effects.When heated to decomposition it emits very toxic fumesof NOx and SOx. See other penicillin entries.
Check Digit Verification of cas no
The CAS Registry Mumber 1538-09-6 includes 7 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 4 digits, 1,5,3 and 8 respectively; the second part has 2 digits, 0 and 9 respectively.
Calculate Digit Verification of CAS Registry Number 1538-09:
(6*1)+(5*5)+(4*3)+(3*8)+(2*0)+(1*9)=76
76 % 10 = 6
So 1538-09-6 is a valid CAS Registry Number.
InChI:InChI=1/2C16H18N2O4S.C16H20N2/c2*1-16(2)12(15(21)22)18-13(20)11(14(18)23-16)17-10(19)8-9-6-4-3-5-7-9;1-3-7-15(8-4-1)13-17-11-12-18-14-16-9-5-2-6-10-16/h2*3-7,11-12,14H,8H2,1-2H3,(H,17,19)(H,21,22);1-10,17-18H,11-14H2/t2*11-,12+,14-;/m11./s1