1.Identification
1.1 GHS Product identifier
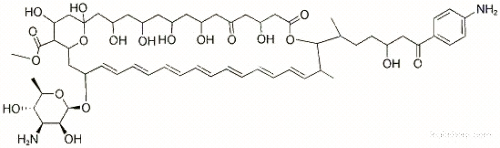
| Product name | Mepartricin |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | MEPARTRICIN |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
1.5 Emergency phone number
| Emergency phone number | - |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |