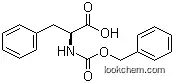
1161-13-3Relevant articles and documents
Selecting optimal conditions for Alcalase CLEA-OM for synthesis of dipeptides in organic media
Vossenberg,Beeftink,Nuijens,Cohen Stuart,Tramper
, p. 43 - 49 (2012)
In protease-catalyzed peptide synthesis, the availability of water is essential, as a compromise must be made between on the one hand the overall enzymatic activity and, on the other hand, the rate of product synthesis. Water is essential for enzyme activity, but at the same time causes hydrolytic side reactions. We studied the coupling of the carbamoylmethyl ester of N-protected phenylalanine and phenylalanine amide in tetrahydrofuran catalyzed by Alcalase CLEA-OM at a range of water activity (aw) values, including the coupling in the presence of molecular sieves (i.e. at very low aw values). The hydrolytic side reaction (in the present system only the hydrolysis of substrate occurs) was found to dominate above an aw value of about 0.2. To prevent hydrolysis, the presence of molecular sieves was found to be necessary.
Peptiligase, an Enzyme for Efficient Chemoenzymatic Peptide Synthesis and Cyclization in Water
Toplak, Ana,Nuijens, Timo,Quaedflieg, Peter J. L. M.,Wu, Bian,Janssen, Dick B.
, p. 2140 - 2147 (2016)
We describe a novel, organic cosolvent-stable and cation-independent engineered enzyme for peptide coupling reactions. The enzyme is a variant of a stable calcium-independent mutant of subtilisin BPN′, with the catalytic Ser212 mutated to Cys and Pro216 converted to Ala. The enzyme, called peptiligase, catalyzes exceptionally efficient peptide coupling in water with a surprisingly high synthesis over hydrolysis (S/H) ratio. The S/H ratio of the peptide ligation reaction is correlated to the length of the peptide substrate and proved to be >100 for the synthesis of a 13-mer peptide, which corresponds to >99% conversion to the ligated peptide product and 1% hydrolytic side-reaction. Furthermore, peptiligase does not require a particular recognition motif resulting in a broadly applicable and traceless peptide ligation technology. Peptiligase is very robust, easy to produce in Bacillus subtilis, and its purification is straightforward. It shows good activity and stability in the presence of organic cosolvents and chelating or denaturing agents, enabling the ligation of poorly soluble (hydrophobic) or folded peptides. This enzyme could be useful for the (industrial) synthesis of diverse (pharmaceutical) peptides. In addition, peptiligase is able to efficiently catalyze head-to-tail peptide cyclization reactions. (Figure presented.) .
Can self-assembled hydrogels composed of aromatic amino acid derivatives function as drug delivery carriers?
Tiwari, Priyanka,Rajagopalan, Ramanathan,Moin, Mohammad,Soni, Rohit,Trivedi, Piyush,DuttKonar, Anita
, p. 308 - 315 (2016)
Low molecular weight hydrogelators (LMOHGs) have attracted recent attention due to their diversified applications. In an attempt to artificially imitate their importance in the design of drug delivery carriers, we have synthesized two simple N-terminally protected aromatic amino-acid derivatives that form efficient stable hydrogels at room temperature. The gelation properties of the hydrogels have been thoroughly investigated using various techniques and their strength has been determined by rheological studies. In order to explore the efficacy of the hydrogels as tools for drug delivery, we have developed hydrogel nanoparticles (HNPs) using a surfactant and high-speed homogenization approach. Interestingly, our hydrogel nanoparticles display good entrapment efficiency and release kinetics of the model drug 5-fluoro uracil from the hydrogel matrix. Our experimental results reveal that hydrogel II displays slightly higher efficiency as a drug delivery carrier, which may be due to the presence of an aromatic ring in the backbone in comparison to hydrogel I. This increased strength may be attributed to the increase in π-π interactions when the aromatic residue is present in the backbone. Therefore the nanoparticles generated from hydrogel II may have better hydrogen bonding abilities with drugs in comparison to hydrogel I; thus resulting in a slightly slower release of drug from the hydrogel matrix. This fact may shed some light on the candidature of our hydrogels as future carriers for drug delivery. However, further studies to evaluate the candidature of these novel types of aromatic amino acid hydrogel nanoparticles for nano-medical applications are under investigation.
Acylation of Alcohols, Amines and Amine Salts with Acid Chlorides in the Presence of Amides
Weisz, Imre,Oetvoes, Laszlo
, p. 766 - 768 (1985)
-
-
Goodman,Stueben
, p. 112 (1959)
-
Bottom-Up Construction of an Adaptive Enzymatic Reaction Network
Helwig, Britta,van Sluijs, Bob,Pogodaev, Aleksandr A.,Postma, Sjoerd G. J.,Huck, Wilhelm T. S.
, p. 14065 - 14069 (2018)
The reproduction of emergent behaviors in nature using reaction networks is an important objective in synthetic biology and systems chemistry. Herein, the first experimental realization of an enzymatic reaction network capable of an adaptive response is reported. The design is based on the dual activity of trypsin, which activates chymotrypsin while at the same time generating a fluorescent output from a fluorogenic substrate. Once activated, chymotrypsin counteracts the trypsin output by competing for the fluorogenic substrate and producing a non-fluorescent output. It is demonstrated that this network produces a transient fluorescent output under out-of-equilibrium conditions while the input signal persists. Importantly, in agreement with mathematical simulations, we show that optimization of the pulse-like response is an inherent trade-off between maximum amplitude and lowest residual fluorescence.
Cobalt-Catalyzed Deprotection of Allyl Carboxylic Esters Induced by Hydrogen Atom Transfer
Li, Nan,Gui, Yizhen,Chu, Mengqi,You, Mengdi,Qiu, Xiaohan,Liu, Hejia,Wang, Shiang,Deng, Meng,Ji, Baoming
supporting information, p. 8460 - 8464 (2021/11/13)
A brief, efficient method has been developed for the removal of the allyl protecting group from allyl carboxylic esters using a Co(II)/TBHP/(Me2SiH)2O catalytic system. This facile strategy displays excellent chemoselectivity, functional group tolerance, and high yields. This transformation probably occurs through the hydrogen atom transfer process, and a Co(III)-six-membered cyclic intermediate is recommended.
Design and synthesis of tripeptidyl furylketones as selective inhibitors against the β5 subunit of human 20S proteasome
Lü, Zirui,Li, Xiaona,Niu, Yan,Sun, Qi,Wang, Chao,Xi, Dandan,Xu, Fengrong,Xu, Ping,Zhou, Tongliang
, (2020/03/10)
A series of tripeptidic proteasome inhibitors with furylketone as C-terminus were designed and synthesized. Biochemical evaluations against β1, β2 and β5 subunits revealed that they acted selectively on β5 subunit with IC50s against chymotrypsin-like (CT-L) activity in micromolar range. LC-MS/MS analysis of the ligand-20S proteasome mixture showed that the most potent compound 11m (IC50 = 0.18 μM) made no covalent modification on 20S proteasome. However, it was identified acting in a slowly reversible manner in wash-out assay and the reversibility was much lower than that of MG132, suggesting the possibility of these tripeptidic furylketones forming reversible covalent bonds with 20S proteasome. Several compounds were selected for anti-proliferative assay towards multiple cancer cell lines, and compound 11m displayed comparable potency to positive control (MG132) in all cell lines tested. Furthermore, the pharmacokinetic (PK) data in rats indicated 11m behaved similarly (Cmax, 2007 μg/L; AUC0?t, 680 μg/L·h; Vss, 0.66 L/kg) to the clinical used agent carfilzomib. All these data suggest 11m is a good lead compound to be developed to novel anti-tumor agent.