156925-25-6Relevant articles and documents
METHODS FOR PREPARING MOSAPRIDE CITRATE HYDRATE AND PHARMACEUTICAL COMPOSITION COMPRISING THE SAME
-
Paragraph 0121-0145, (2020/10/19)
The present invention relates to a method for preparing a salt hydrate of mosapride and a pharmaceutical formulation comprising the same. The present invention relates to a method for manufacturing a semiconductor device. A pharmaceutical composition for controlled release and a pharmaceutical composition containing the same are provided to have little side effect, little side effect with other drugs or excipients, and have excellent physical properties for use in a pharmaceutical composition containing the controlled release pharmaceutical composition. (by machine translation)
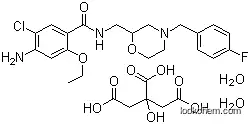
Citric acid mosapride intermediate product and application
-
, (2018/09/08)
The invention belongs to the field of medical chemistry synthesis, and provides a preparation method of citric acid mosapride intermediate product IV 4-[(4-fluorophenyl)methyl]-2-morpholinemethanaminesalt and citric acid mosapride. The 2-(4-fluorobenzoamido)ethanol and 1H-Isoindole-1,3(2H)-dione,2-(2-oxiranylmethyl) are taken as raw materials, and the intermediate product IV 4-[(4-fluorophenyl)methyl]-2-morpholinemethanamine salt is obtained after acid treating is conducted; the intermediate product IV and an intermediate V 2-oxethyl-4-acetamido-5-Chlorobenzoic acid ethyl ester compounds aretaken as raw materials, dichloromethane is taken as a solvent, and EDCI and DMAP are taken as catalysts to prepare mosapride salt; the mosapride salt is reacted with citric acid aqueous solution to prepare citric acid mosapride. The intermediate product has the advantages that products are high in yield, raw materials are easy to obtain, the production cost is low, and the intermediate product issuitable for industrialized production.
HIGHLY PURE MOSAPRIDE CITRATE DIHYDRATE AND PROCESSES FOR ITS PREPARATION
-
Page/Page column 11, (2011/10/03)
The present invention provides for highly pure mosapride citrate dihydrate and processes for its preparation. The present invention further provides a process for the preparation of mosapride citrate dihydrate substantially free of impurity D-II.