188627-80-7 Usage
Uses
Used in Cardiology:
Eptifibatide is used as an anticoagulant for the treatment of acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI). It helps in reducing the risk of acute cardiac ischemic events in patients with unstable angina or non-ST segment-elevation myocardial infarction. It is often used in combination with aspirin or clopidogrel and heparin.
Used in Pharmaceutical Development:
Eptifibatide is used as a research compound for the development of improved antithrombotic agents. Due to its short half-life, it is required to be improved with a protected and targeted delivery system, such as using nano-liposomes to the site of thrombus, to enhance its efficacy and bioavailability.
References
https://www.drugbank.ca/drugs/DB00063
http://www.rxlist.com/integrilin-drug/indications-dosage.htm
https://en.wikipedia.org/wiki/Eptifibatide
Originator
Cor Therapeutics (US)
Biological Activity
Eptifibatide is a potent glycoprotein IIb/IIIa antagonist (GPIIb/IIIa; Kd = 120 nM) that inhibits platelet aggregation. Eptifibatide prevents binding of the adhesion proteins fibrinogen and von Willebrand factor to GPIIb/IIIa on the surface of activated platelets to prevent aggregation and thrombus formation. It inhibits ADP-induced citrated blood aggregation (IC50 = 0.11-0.22 μg/ml) in vitro and in vivo (IC50 = 52 μg/ml in porcine plasma). Formulations containing eptifibatide have been used to reduce risk of thrombolysis in myocardial infarction in patients undergoing percutaneous coronary intervention.
Mechanism of action
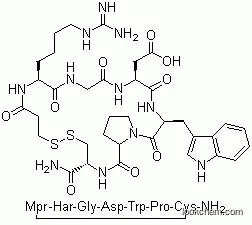
Eptifibatide, a synthetic cyclic heptapeptide containing six amino acids, including one cysteine amide and one mercaptopropionyl (desamino cysteinyl) residue, is an inhibitor of platelet aggregation and belongs to the class of RGD (arginine-glycine-aspartate)-mimetics.Eptifibatide reversibly inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor and other adhesive ligands to the glycoprotein (GP) IIb/IIIa receptors.
Clinical Use
Eptifibatide is a cyclic heptapeptide composed of six amino acids and one mercaptopropionyl
residue. The cyclization is completed via a disulfide linkage between the cysteine and the
mercaptopropionyl moieties. The lysine-glycine-aspartate component of eptifibatide is highly specific
for the GPIIb/IIIa receptor, with low binding affinity, as indicated by the rapid dissociation constant. Because of this,
eptifibatide is a reversible, parenterally administered antagonist of platelet aggregation.
Drug interactions
Potentially hazardous interactions with other drugs
Iloprost: increased risk of bleeding.
Metabolism
Renal excretion accounts for approximately 50% of total
body clearance of eptifibatide; approximately 50% of the
amount cleared is excreted unchanged in the urine.
Check Digit Verification of cas no
The CAS Registry Mumber 188627-80-7 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 1,8,8,6,2 and 7 respectively; the second part has 2 digits, 8 and 0 respectively.
Calculate Digit Verification of CAS Registry Number 188627-80:
(8*1)+(7*8)+(6*8)+(5*6)+(4*2)+(3*7)+(2*8)+(1*0)=187
187 % 10 = 7
So 188627-80-7 is a valid CAS Registry Number.
InChI:InChI=1/C35H49N11O9S2/c36-31(52)26-17-57-56-12-10-27(47)43-22(7-3-4-11-39-35(37)38)32(53)41-16-28(48)44-25(14-29(49)50)34(55)45-24(13-18-15-40-20-6-2-1-5-19(18)20)30(51)21-8-9-23(42-21)33(54)46-26/h1-2,5-6,15,21-26,40,42H,3-4,7-14,16-17H2,(H2,36,52)(H,41,53)(H,43,47)(H,44,48)(H,45,55)(H,46,54)(H,49,50)(H4,37,38,39)/t21?,22-,23-,24-,25-,26-/m0/s1
188627-80-7Relevant articles and documents
PROCESS FOR PREPARING EPTIFIBATIDE
-
Page/Page column, (2014/06/24)
The present invention provides processes for preparation of eptifibatide that involve coupling of amino acids in a (2+5), (4+3) and (3+4) sequence method. The invention further provides products produced by the described processes, novel compounds that can be used as synthetic intermediates for the preparation of eptifibatide.
Novel diphenylmethyl-derived amide protecting group for efficient liquid-phase peptide synthesis: AJIPHASE
Takahashi, Daisuke,Yano, Tatsuya,Fukui, Tatsuya
supporting information, p. 4514 - 4517 (2012/10/29)
An efficient method for the synthesis of peptides bearing an amide at the C-terminal is described. This method involves the attachment of a C-terminal protecting group bearing long aliphatic chains, followed by the repetition of simple reaction and precipitation steps with the combined advantages of liquid-phase peptide synthesis (LPPS) and solid-phase peptide synthesis (SPPS). Using this method, a hydrophobic peptide was successfully synthesized in good yield and high purity, which cannot be obtained satisfactorily by SPPS.