203120-17-6 Usage
Description
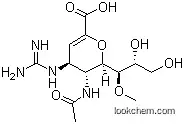
Laninamivir octanoate (CS-8958), an ester prodrug form of the
neuraminidase (NA) inhibitor laninamivir (R-125489), was approved in
Japan in 2010 for treatment of influenza virus infections. Laninamivir
octanoate is given by intranasal administration at a 20 mg or 40 mg dose.
It has a long half-life in humans such that efficacy can be achieved after
only a single dose. In addition to vaccines for immunoprophylaxis, antiviral drugs play an
essential role in the treatment of influenza virus infections. Two
viral proteins have been targeted for therapeutic intervention: the M2
ion channel and NA.
Originator
Sankyo Co., Ltd. (Japan)
Uses
Different sources of media describe the Uses of 203120-17-6 differently. You can refer to the following data:
1. A new potent neuraminidase (NA) inhibitor, shows long-acting anti-influenza virus activity.
2. A new potent labelled neuraminidase
3. A new potent neuraminidase
Brand name
Inavir
Synthesis
Laninamivir octanoate is prepared starting from a neuraminic acid precursor. The route from 2,3-didehydroneuramic acid entails a multistep sequence to protect the acid and hydroxyl groups at the 4-, 20-, and 30-positions. Methylation of the remaining 10-hydroxyl by treatment with dimethylsulfate and NaH is followed by conversion of the 4-hydroxyl to an amine Cleavage of the 20,30-dihydroxy protecting group, conversion of the 4- NH2 to the guanidine, and acylation of the 30-OH group afford laninamivir octanoate. This three-step sequence can be reordered such that the guanidine is introduced first, followed by deprotection of the 20,30-diOH groups and acylation. An alternative sequence involves a Boc-protected guanidine intermediate, which is converted in a four-step sequence (deprotection of the acid and 20,30-hydroxyl groups, reprotection of the acid as its diphenylmethyl ether, acylation of the 30-OH and deprotection of the guanidine group) to laninamivir octanoate. Laninamivir can also be synthesized from the a-methyl glycoside of N-acetylneuramic acid methyl ester by an analogous route.
Check Digit Verification of cas no
The CAS Registry Mumber 203120-17-6 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 2,0,3,1,2 and 0 respectively; the second part has 2 digits, 1 and 7 respectively.
Calculate Digit Verification of CAS Registry Number 203120-17:
(8*2)+(7*0)+(6*3)+(5*1)+(4*2)+(3*0)+(2*1)+(1*7)=56
56 % 10 = 6
So 203120-17-6 is a valid CAS Registry Number.
InChI:InChI=1/C13H22N4O7/c1-5(19)16-9-6(17-13(14)15)3-8(12(21)22)24-11(9)10(23-2)7(20)4-18/h3,6-7,9-11,18,20H,4H2,1-2H3,(H,16,19)(H,21,22)(H4,14,15,17)/t6-,7+,9+,10+,11+/m0/s1
203120-17-6Relevant articles and documents
Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives.
Masuda, Takeshi,Yoshida, Shuku,Arai, Masami,Kaneko, Satoru,Yamashita, Makoto,Honda, Takeshi
, p. 1386 - 1398 (2003)
Polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives on a poly-L-glutamine backbone are described. Aiming for a longer retention time of 4-guanidino-Neu5Ac2en (zanamivir) in bronchi and lungs, we focused on supermolecules bearing 4-gu
METHOD FOR PRODUCING NEURAMINIC ACID DERIVATIVE
-
, (2014/10/29)
The present invention provides methods for manufacturing neuraminic acid derivatives. [Means for solution] Methods for manufacturing compounds represented by the formula (I) : [wherein R1 represents a C1-C19 alkyl group], or a pharmacologically acceptable salt thereof, using N-acetylneuraminic acid dihydrate as a starting raw material are provided.
METHOD FOR MANUFACTURING NEURAMINIC ACID DERIVATIVES
-
Page/Page column 77, (2008/12/08)
A method for manufacturing neuraminic acid derivatives is provided, also synthetic intermediates of the neuraminic acid derivatives and methods for their manufacture, and neuraminic acid derivatives having high purity. [Means for solution] A synthetic intermediate compound represented by the formula (7) is provided: [wherein R3 represents alkyl; R4 and R5 each represents H, alkyl, phenyl, or together represent tetramethylene, pentamethylene, oxo].