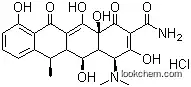
24390-14-5 Usage
Description
Doxycycline Hyclate is the hyclate salt form of doxycycline, is a broad-spectrum tetracycline antibiotic. It inhibits bacterial protein synthesis by binding to ribosomes. Doxycycline also selectively inhibits human matrix metalloproteinase-8 (MMP-8) and MMP-13 over MMP-1 with 50, 60, and 5% inhibition, respectively, when used at a concentration of 30 μM. It can be used as a regulator for inducible gene expression systems where expression depends on either the presence (Tet-On) or absence (Tet-Off) of doxycycline. Formulations containing doxycycline have been used in the treatment of bacterial infections and the prevention of malaria.
Chemical Properties
Doxycycline hyclate is a yellow, hygroscopic crystalline powder, freely soluble in water and in methanol, sparingly soluble in ethanol (96 per cent), it dissolves in solutions of alkali hydroxides and carbonates.
Uses
Doxycycline hyclate is a member of the tetracycline antibiotics group, and is commonly used to treat a variety of infections. It is used in the treatment of chlamydia, rickettsia, mycoplasma and some spirochete infections. It is also used to inhibit matrix metalloproteinases at subantimicrobial doses. Inhibits matrix metalloproteinases at subantimicrobial doses.
Definition
ChEBI: The hemiethanolate hemihydrate of doxycycline hydrochloride. A semi-synthetic tetracycline antibiotic, it is used to inhibit bacterial protein synthesis and treat non-gonococcal urethritis and cervicitis, exacerbations of bronchitis in patients with chroni
obstructive pulmonary disease (COPD), and adult periodontitis.
Application
Doxycycline hyclate has been used for the induction of glioma cells and human embryonic kidney 293T cells (HEK293T).Doxycycline hyclate is a synthetic oxytetracycline derivative. It has been used to eliminate Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and to eliminate Ixodes scapularis ticks. It is a broad spectrum inhibitor used to inhibit matrix metalloproteinases(MMP), such as type 1 collagenase in studies on wound healing and tissue remodeling.
Brand name
Atridox (QLT); Doryx (Faulding); Doryx (Warner Chilcott); Doxy (Abraxis); Doxychel Hyclate (Rachelle); Periostat (CollaGenex); Vibramycin (Pfizer);Abadox;Bassado;Bio-tab;Clisemina;Cloran;Cyclidox;Diocimex;Docostyl;Dosil;Dotur;Doxatet;Doxi sergo;Doxicento;Doxifin;Doxilen;Doximycin;Doxiten bio;Doxivis;Doxy-100;Doxy-basan;Doxybiocin;Doxycyl;Doxy-diolan;Doxydyn;Doxyfim;Doxygram;Doxylag;Doxylan;Doxylar;Doxylets;Doxymycin;Doxy-p;Doxytem;Doxy-wolff;Dumoxin;Duradoxal;Esadoxi;Farmodoxi;Ghimadox;Gram-val;Granudoxy;Helvedoclyn;Icladox;Miraclin;Monocline;Monodoxin;Novelciclina;Philcociclina;Roxyne;Semelciclina;Sigadoxin;Solupen;Stamicina;Supracyclin;Tetrasan;Unacil;Vibracina;Vibramicina;Vibramycin hyclate;Vibraveineuse;Vibravenosa;Ximicina;Zadorin.
World Health Organization (WHO)
Doxycycline, a semi-synthetic tetracycline derivative, was first
introduced into medicine in 1960 for the treatment of bacterial, rickettsial and
amoebic infections. Although allergic manifestations are uncommon, injectable
preparations have occasionally resulted in severe anaphylactoid reactions. Clinical
features and the fact that asthmatic patients seemed to be particularly at risk lead
to suspect a sulfite preservative in the formulation more than doxycycline itself.
Rapid administration may also be a factor. Injectable preparations of doxycycline
hyclate are included in the WHO Model List of Essential Drugs.
General Description
Doxycycline hyclate belongs to the second generation of tetracycline antibiotics family. Doxycycline hyclate shows minimal side effects upon usage. It is effective for the treatment of recurrent aphthous stomatitis (RAS) and promotes speedy recovery.
Biochem/physiol Actions
Doxycycline hyclate inhibits the inflammatory response to the Lyme Disease Spirochete Borrelia burgdorferi. It is a broad spectrum inhibitor of matrix metalloproteinases in vivo.
Mode of action
Tetracycline antimicrobials bind to the bacterial 30S ribosomal subunit interfering with tRNA/mRNA interaction, ultimately inhibiting protein synthesis. Tetracyclines can inhibit the MMP enzyme family and inhibit mitochondrial biogenesis.
Check Digit Verification of cas no
The CAS Registry Mumber 24390-14-5 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 2,4,3,9 and 0 respectively; the second part has 2 digits, 1 and 4 respectively.
Calculate Digit Verification of CAS Registry Number 24390-14:
(7*2)+(6*4)+(5*3)+(4*9)+(3*0)+(2*1)+(1*4)=95
95 % 10 = 5
So 24390-14-5 is a valid CAS Registry Number.
InChI:InChI=1/C22H24N2O8.ClH/c1-7-8-5-4-6-9(25)11(8)16(26)12-10(7)17(27)14-15(24(2)3)18(28)13(21(23)31)20(30)22(14,32)19(12)29;/h4-7,10,14-15,17,25,27-29,32H,1-3H3,(H2,23,31);1H/t7-,10+,14+,15-,17-,22-;/m0./s1