286930-03-8 Usage
Description
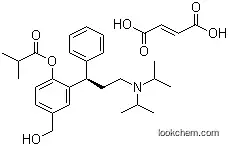
Fesoterodine fumarate is a new drug for the treatment of overactive bladder syndrome developed by Pfizer, which was approved by the US FDA in October 2008. Fesoterodine fumarate is a prodrug, which is rapidly hydrolyzed in blood after oral administration to 5-hydroxymethyl tolterodine (5-HMT), which is also the active metabolite of tolterodine.
Biological activity
Fesoterodine Fumarate (SPM 907) is a prodrug of the muscarinic receptor antagonist 5-hydroxymethyl tolterodine, used to treat overactive bladder.
Side effects
The most common side effects was dry mouth, which was observed in the placebo group, the product 4 in the Phase II and Phase III clinical trials (a total of 2 859 patients, 2 288 taking this product for 8 to 12 weeks). The incidences of dry mouth at mg/d and 8 mg/d were 7%, 19%, and 35%, respectively, and the incidences of drug discontinuation due to dry mouth were 0.4%, 0.4%, and 0.8%, respectively. The second most common adverse reaction was constipation. The incidence of constipation in the placebo group and the 4 mg/d and 8 mg/d groups of this product was 2%, 4% and 6%, respectively. Other reported adverse events included loss of appetite, nausea, epigastric pain, urinary tract infection, upper respiratory tract infection, dry eyes, dysuria, urinary retention, cough, peripheral edema, back pain, insomnia, abnormal liver function, and rash.
Chemical Properties
White Solid
Uses
Different sources of media describe the Uses of 286930-03-8 differently. You can refer to the following data:
1. Fesoterodine fumarate (Toviaz) is an antimuscarinic agent and is rapidly de-esterified to its active metabolite 5-hydroxymethyl tolterodine that is a muscarinic receptor antagonist. Fesoterodine fumarate (Toviaz) is used to treat the symptoms of overactiv
2. (R)-Fesoterodine Fumarate is a muscarinic receptor antagonist for the treatment of Lower Urininary Tract Symptoms (LUTS). It is very similar to Tolterodine (T535800).
Clinical Use
Antimuscarinic:Symptomatic treatment of urinary incontinence,
frequency or urgency
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased risk of antimuscarinic
side effects with disopyramide.
Antifungals: dose reduction advised with
itraconazole and ketoconazole.
Antivirals: dose reduction advised with atazanavir,
indinavir, ritonavir and saquinavir.
Induction of CYP3A4 may lead to subtherapeutic
plasma levels. Concomitant use with CYP3A4
inducers (e.g. carbamazepine, rifampicin,
phenobarbital, phenytoin, St John's Wort) is not
recommended.
Co-administration of a potent CYP2D6 inhibitor
may result in increased exposure and adverse events.
A dose reduction to 4 mg may be needed'
See 'Other information'
Metabolism
Molecular weight (daltons) 527.7
% Protein binding 50 (metabolite)
% Excreted unchanged in urine 70 (as metabolites)
Volume of distribution (L/kg) 169 Litres
Half-life - normal/ESRF (hrs) 7 / -
Check Digit Verification of cas no
The CAS Registry Mumber 286930-03-8 includes 9 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 6 digits, 2,8,6,9,3 and 0 respectively; the second part has 2 digits, 0 and 3 respectively.
Calculate Digit Verification of CAS Registry Number 286930-03:
(8*2)+(7*8)+(6*6)+(5*9)+(4*3)+(3*0)+(2*0)+(1*3)=168
168 % 10 = 8
So 286930-03-8 is a valid CAS Registry Number.
InChI:InChI=1/C26H37NO3.C4H4O4/c1-18(2)26(29)30-25-13-12-21(17-28)16-24(25)23(22-10-8-7-9-11-22)14-15-27(19(3)4)20(5)6;5-3(6)1-2-4(7)8/h7-13,16,18-20,23,28H,14-15,17H2,1-6H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t23-;/m1./s1
286930-03-8Relevant articles and documents
ANTIMUSCARINIC COMPOUND HAVING A LOW CONTENT OF IMPURITIES
-
Paragraph 0061, (2016/02/20)
Substantially stable to degradation Fesoterodine fumarate, a process for its preparation and a process for the synthesis of specific degradation impurities of Fesoterodine fumarate are disclosed.
PROCESS FOR THE PREPARATION OF 2-(3-N,N-DIISOPROPYLAMINO-1-PHENYLPROPYL)-4-HYDROXYMETHYL-PHENOL AND ITS DERIVATIVES
-
Page/Page column 49-50, (2014/02/15)
The invention concerns a process for the preparation of 2-(3-N,N- diisopropylamino-l-phenylpropyl)-4-hydroxymethyl-phenol and its derivatives, particularly the corresponding (R) 4-trityloxymethyl derivative, useful as intermediate form in the synthesis of Fesoterodine and its salts, in particular for the preparation of Fesoterodine fumarate salt.
PROCESS FOR THE PREPARATION OF OPTICALLY ACTIVE 3,3-DIPHENYLPROPYLAMINES
-
, (2013/08/15)
The invention relates to a process for obtaining 3,3-diphenylpropylamines of general formula (I), particularly Fesoterodine, as well as their enantiomers, solvates and salts, comprising treating a compound of formula (II) with a chiral alcohol to yield the diastereomeric esters of formula (IV) and (IV'), which can be further transformed into a compound of formula (I), or an enantiomer, solvate or salt thereof, wherein R1 is C1-C8 alkyl; and R2 and R3, independently of one another, represent H or C1-C6 alkyl, or together form a ring of 3 to 7 members with the nitrogen to which they are bound.