687603-66-3Relevant articles and documents
Chiral resolution and absolute configuration of the enantiomers of the psychoactive designer drug 3,4-methylenedioxypyrovalerone
Suzuki, Masaki,Deschamps, Jeffrey R.,Jacobson, Arthur E.,Rice, Kenner C.
, p. 287 - 293 (2015)
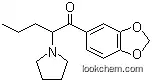
Illicit rac-MDPV (3,4-methylenedioxypyrovalerone), manufactured in clandestine labs, has become widely abused for its cocaine-like stimulant properties. It has recently been found as one of the toxic materials in the so-called bath salts, producing, among other effects, psychosis and tachycardia in humans when introduced by any of the several routes of administration (e.g., intravenous, oral, etc.). The considerable toxicity of this designer drug probably resides in one of the enantiomers of the racemate. In order to obtain a sufficient amount of the enantiomers of rac-MDPV to determine their activity, we improved the known synthesis of rac-MDPV and found chemical resolving agents, (+)- and (-)-2'-bromotetranilic acid, that gave the MDPV enantiomers in >96% enantiomeric excess as determined by 1H nuclear magnetic resonance and chiral high-performance liquid chromatography. The absolute stereochemistry of these enantiomers was determined by single-crystal X-ray diffraction studies. Chirality 27:287-293, 2015. Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.