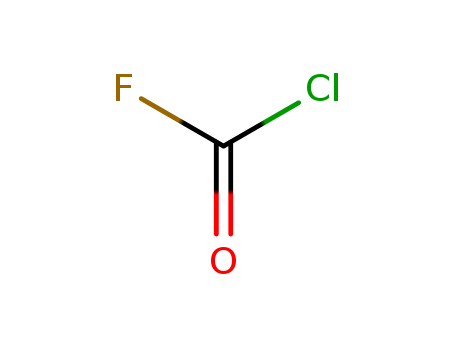
- Chemical Name:Carbonyl chloride fluoride
- CAS No.:353-49-1
- Molecular Formula:CClFO
- Molecular Weight:82.4618
- Hs Code.:
- European Community (EC) Number:206-533-7
- DSSTox Substance ID:DTXSID8073897
- Nikkaji Number:J193.825H
- Wikidata:Q82002483
- Mol file:353-49-1.mol
Synonyms:Carbonyl chloride fluoride;Carbonic chloride fluoride;353-49-1;EINECS 206-533-7;CARBONYLCHLORIDEFLUORIDE;CClFO;COClF;Fluorochloromethanone;Carbonyl chlorofluoride;C-Cl-F-O;Carbonyl chloride fluoride 97%;DTXSID8073897;MFCD00042118;Estra-1,3,5(10), 7-tetraen-17-one, 3-hydroxy-;Cholan-24-oic acid, 3,7-dihydroxy-, (3a,5.beta.,7a)-



 C;
C;  F
F


