Base Information
Edit
- Chemical Name:Gengraf
- CAS No.:79217-60-0
- Molecular Formula:C62H111N11O12
- Molecular Weight:1202.61
- Hs Code.:
- European Community (EC) Number:611-907-1
- Wikipedia:Ciclosporin
- NCI Thesaurus Code:C406
- RXCUI:3008
- Mol file:79217-60-0.mol
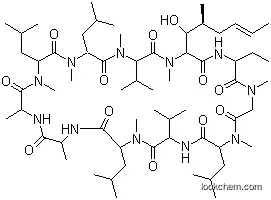
Synonyms:Ciclosporin;CsA Neoral;CsA-Neoral;CsANeoral;CyA NOF;CyA-NOF;Cyclosporin;Cyclosporin A;Cyclosporine;Cyclosporine A;Neoral;OL 27 400;OL 27-400;OL 27400;Sandimmun;Sandimmun Neoral;Sandimmune


 T,
T, Xn
Xn
