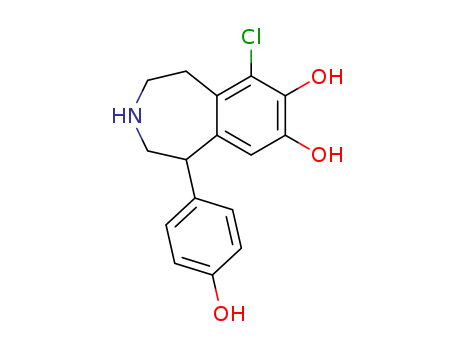
- Chemical Name:Fenoldopam
- CAS No.:67227-56-9
- Deprecated CAS:87900-90-1
- Molecular Formula:C16H16ClNO3
- Molecular Weight:305.761
- Hs Code.:
- UNII:INU8H2KAWG
- DSSTox Substance ID:DTXSID0043896
- Nikkaji Number:J23.754J
- Wikipedia:Fenoldopam
- Wikidata:Q2357007
- NCI Thesaurus Code:C61759
- RXCUI:24853
- Pharos Ligand ID:8JM1SAV8DS1Y
- Metabolomics Workbench ID:43091
- ChEMBL ID:CHEMBL588
- Mol file:67227-56-9.mol
Synonyms:Corlopam;Fenoldopam;Fenoldopam Hydrobromide;Fenoldopam Mesylate;Hydrobromide, Fenoldopam;SK and F 82526;SK and F 82526J;SK and F-82526;SK and F-82526J;SK and F82526;SK and F82526J;SKF 82526;SKF 82526J;SKF-82526;SKF-82526J;SKF82526;SKF82526J