- Chemical Name:Barium sulfide
- CAS No.:21109-95-5
- Deprecated CAS:97560-32-2
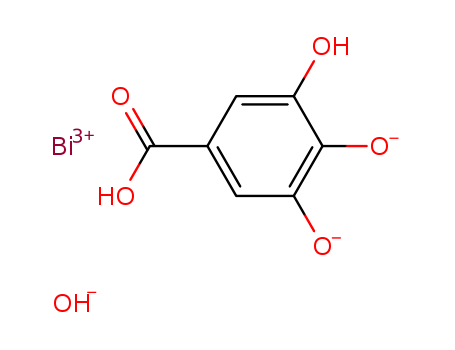
- Molecular Formula:Bi*C7H4O5*HO
- Molecular Weight:394.094
- Hs Code.:
- European Community (EC) Number:232-376-9,256-814-3
- UNII:TV3U2GEW4H
- DSSTox Substance ID:DTXSID30892157
- Wikipedia:Barium sulfide,Barium_sulfide
- Wikidata:Q411656
- Mol file:21109-95-5.mol
Synonyms:Bariummonosulfide;Barium sulfide (BaS);


 Xn,
Xn,  Xi,
Xi,  N
N
 Xn:Harmful;
Xn:Harmful;

